Treatment response assessment to chemotherapy with bevacizumab for colorectal liver metastasis by contrast-enhanced ultrasound
- PMID: 38223071
- PMCID: PMC10784026
- DOI: 10.21037/qims-23-1027
Treatment response assessment to chemotherapy with bevacizumab for colorectal liver metastasis by contrast-enhanced ultrasound
Abstract
Background: Though contrast-enhanced ultrasound (CEUS) perfusion parameters have been approved to be potential indicators for response to chemotherapy in solid tumors, their ability in assessment of colorectal liver metastasis (CRLM) to chemotherapy with bevacizumab (Bev) has rarely been investigated.
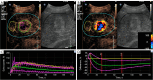
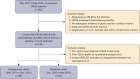
Methods: From March 2021 to May 2022, 115 consecutive CRLM patients with CEUS pre- and post-2 months' chemotherapy with Bev were prospectively enrolled. One target lesion per patient underwent CEUS quantitative analysis with SonoLiver software. Rise time, time-to-peak, mean transit time, maximal intensity (IMAX), and area under the time-intensity curve (AUC) were assessed with region of interest (ROI) selected on whole lesion, lesion periphery, and internal lesion, respectively. The reduction and ratio of post- to pre-treatment in parameters were investigated in development cohort (n=89) and validated in internal validation cohort (n=26) according to the chronological order.
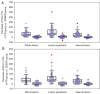
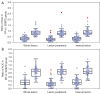
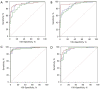
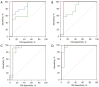
Results: With modified Response Evaluation Criteria in Solid Tumor as reference, 48, 14 responders and 41, 12 non-responders were included in development and validation cohort, respectively. Significantly smaller values of IMAX and AUC on ROIwhole, ROIperipheral, and ROIinternal, were observed post-treatment in development cohort (all P<0.05). In predicting treatment response, the influence of ROI selection was observed when using ∆IMAX and ∆AUC, while no influence was observed using ratios. Areas under the receiver operating characteristic curve (AUROCs) for ∆IMAX and ∆AUC on ROIperipheral were 0.939 (0.867-0.979), 0.951 (0.883-0.985), and 0.917 (0.740-0.988), 0.923 (0.748-0.990) in development and validation cohort, respectively. For ratios of IMAX and AUC, AUROCs were 0.976 (0.919-0.997), 0.938 (0.865-0.978), and 0.899 (0.717-0.982), 0.982 (0.836-1.000) in development and validation cohort, respectively.
Conclusions: IMAX and AUC showed significant reductions in responders, and different analyses ROIs influence the performance of ∆IMAX and ∆AUC in response assessment. Parameters derived from ROI peripheral exhibited the most promising results in predicting treatment response.
Keywords: Contrast-enhanced ultrasound (CEUS); colorectal carcinoma; liver metastasis; response.
2024 Quantitative Imaging in Medicine and Surgery. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1027/coif). The authors have no conflicts of interest to declare.
Figures
References
-
- Cardona K, Mastrodomenico P, D'Amico F, Shia J, Gönen M, Weiser MR, Paty PB, Kingham TP, Allen PJ, De Matteo RP, Fong Y, Jarnagin WR, D'Angelica MI. Detailed pathologic characteristics of the primary colorectal tumor independently predict outcome after hepatectomy for metastases. Ann Surg Oncol 2013;20:148-54. 10.1245/s10434-012-2540-y - DOI - PubMed
-
- Imai K, Allard MA, Benitez CC, Vibert E, Sa Cunha A, Cherqui D, Castaing D, Bismuth H, Baba H, Adam R. Early Recurrence After Hepatectomy for Colorectal Liver Metastases: What Optimal Definition and What Predictive Factors? Oncologist 2016;21:887-94. 10.1634/theoncologist.2015-0468 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
