Pharmacokinetics and tissue distribution of vigabatrin enantiomers in rats
- PMID: 38223203
- PMCID: PMC10787297
- DOI: 10.1016/j.jsps.2023.101934
Pharmacokinetics and tissue distribution of vigabatrin enantiomers in rats
Abstract
Purpose: To investigate the pharmacokinetics and tissue distribution of VGB racemate and its single enantiomers, and explore the potential of clinic development for single enantiomer S-VGB.
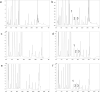
Methods: In the pharmacokinetics study, male Sprague-Dawley rats were gavaged with VGB racemate or its single enantiomers dosing 50, 100 or 200 mg/kg, and the blood samples were collected during 12 h at regular intervals. In the experiment of tissue distribution, VGB and its single enantiomers were administered intravenously dosing 200 mg/kg, and the tissues including heart, liver, spleen, lung and kidney, eyes, hippocampus, and prefrontal cortex were separated at different times. The concentrations of R-VGB and S-VGB in the plasma and tissues were measured using HPLC.
Results: Both S-VGB and R-VGB could be detected in the plasma of rats administered with VGB racemate, reaching Cmax at approximately 0.5 h with t1/2 2-3 h. There was no significant pharmacokinetic difference between the two enantiomers when VGB racemate was given 200 mg/kg and 100 mg/kg. However, when given at the dose of 50 mg/kg, S-VGB presented a shorter t1/2 and a higher Cl/F than R-VGB, indicating a faster metabolism of S-VGB. Furthermore, when single enantiomer was administered respectively, S-VGB presented a slower metabolism than R-VGB, as indicated by a longer t1/2 and MRT but a lower Cmax. Moreover, compared with the VGB racemate, the single enantiomers S-VGB and R-VGB had shorter t1/2 and MRT, higher Cmax and AUC/D, and lower Vz/F and Cl/F, indicating the stronger oral absorption and faster metabolism of single enantiomer. In addition, regardless of VGB racemate administration or single enantiomer administration, S-VGB and R-VGB had similar characteristics in tissue distribution, and the content of S-VGB in hippocampus, prefrontal cortex and liver was much higher than that of R-VGB.
Conclusions: Although there is no transformation between S-VGB and R-VGB in vivo, those two enantiomers display certain disparities in the pharmacokinetics and tissue distribution, and interact with each other. These findings might be a possible interpretation for the pharmacological and toxic effects of VGB and a potential direction for the development and optimization of the single enantiomer S-VGB.
Keywords: Distribution; Pharmacokinetics; Single enantiomer; Vigabatrin.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Al-Majed A.A. A direct HPLC method for the resolution and quantitation of the R-(-)- and S-(+)-enantiomers of vigabatrin (gamma-vinyl-GABA) in pharmaceutical dosage forms using teicoplanin aglycone chiral stationary phase. J. Pharm. Biomed. Anal. 2009;50(1):96–99. doi: 10.1016/j.jpba.2009.03.030. - DOI - PubMed
LinkOut - more resources
Full Text Sources

