Conductive 3D nano-biohybrid systems based on densified carbon nanotube forests and living cells
- PMID: 38223564
- PMCID: PMC10784361
- DOI: 10.1557/s43578-023-01163-x
Conductive 3D nano-biohybrid systems based on densified carbon nanotube forests and living cells
Abstract
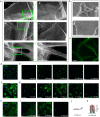
Conductive biohybrid cell-material systems have applications in bioelectronics and biorobotics. To date, conductive scaffolds are limited to those with low electrical conductivity or 2D sheets. Here, 3D biohybrid conductive systems are developed using fibroblasts or cardiomyocytes integrated with carbon nanotube (CNT) forests that are densified due to interactions with a gelatin coating. CNT forest scaffolds with a height range of 120-240 µm and an average electrical conductivity of 0.6 S/cm are developed and shown to be cytocompatible as evidenced from greater than 89% viability measured by live-dead assay on both cells on day 1. The cells spread on top and along the height of the CNT forest scaffolds. Finally, the scaffolds have no adverse effects on the expression of genes related to cardiomyocyte maturation and functionality, or fibroblast migration, adhesion, and spreading. The results show that the scaffold could be used in applications ranging from organ-on-a-chip systems to muscle actuators.
Keywords: Carbon nanotube forest; Cardiomyocytes; Cell scaffold; Conductive nano-biohybrid systems; Densification; Fibroblasts; Gelatin.
© The Author(s) 2023.
Conflict of interest statement
Conflict of interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
Aligned carbon nanotube-based flexible gel substrates for engineering bio-hybrid tissue actuators.Adv Funct Mater. 2015 Jul 20;25(28):4486-4495. doi: 10.1002/adfm.201501379. Epub 2015 Jun 12. Adv Funct Mater. 2015. PMID: 27134620 Free PMC article.
-
Moldable elastomeric polyester-carbon nanotube scaffolds for cardiac tissue engineering.Acta Biomater. 2017 Apr 1;52:81-91. doi: 10.1016/j.actbio.2016.12.009. Epub 2016 Dec 8. Acta Biomater. 2017. PMID: 27940161
-
Carbon nanotube nanocomposite scaffolds: advances in fabrication and applications for tissue regeneration and cancer therapy.Front Bioeng Biotechnol. 2023 Dec 21;11:1299166. doi: 10.3389/fbioe.2023.1299166. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 38179128 Free PMC article. Review.
-
Electrospun conductive nanofibrous scaffolds for engineering cardiac tissue and 3D bioactuators.Acta Biomater. 2017 Sep 1;59:68-81. doi: 10.1016/j.actbio.2017.06.036. Epub 2017 Jun 27. Acta Biomater. 2017. PMID: 28663141
-
Carbon nanotube scaffolds as emerging nanoplatform for myocardial tissue regeneration: A review of recent developments and therapeutic implications.Biomed Pharmacother. 2018 Aug;104:496-508. doi: 10.1016/j.biopha.2018.05.066. Epub 2018 May 25. Biomed Pharmacother. 2018. PMID: 29800914 Review.
References
-
- Compaan AM, et al. Cross-linkable microgel composite matrix bath for embedded bioprinting of perfusable tissue constructs and sculpting of solid objects. ACS Appl. Mater. Interfaces. 2020;12(7):7855–7868. - PubMed
-
- Li S, et al. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat. Biotechnol. 2018;36(3):258–264. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
