Effects of Korean red ginseng on T-cell repopulation after autologous hematopoietic stem cell transplantation in childhood cancer patients
- PMID: 38223820
- PMCID: PMC10785244
- DOI: 10.1016/j.jgr.2023.09.001
Effects of Korean red ginseng on T-cell repopulation after autologous hematopoietic stem cell transplantation in childhood cancer patients
Abstract
Background: Although the survival outcomes of childhood cancer patients have improved, childhood cancer survivors suffer from various degrees of immune dysfunction or delayed immune reconstitution. This study aimed to investigate the effect of Korean Red Ginseng (KRG) on T cell recovery in childhood cancer patients who underwent autologous hematopoietic stem cell transplantation (ASCT) from the perspective of inflammatory and senescent phenotypes.
Methods: This was a single-arm exploratory trial. The KRG group (n = 15) received KRG powder from month 1 to month 12 post-ASCT. We compared the results of the KRG group with those of the control group (n = 23). The proportions of T cell populations, senescent phenotypes, and cytokine production profiles were analyzed at 1, 3, 6, and 12 months post-ASCT using peripheral blood samples.
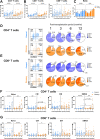
Results: All patients in the KRG group completed the treatment without any safety issues and showed a comparable T cell repopulation pattern to that in the control group. In particular, KRG administration influenced the repopulation of CD4+ T cells via T cell expansion and differentiation into effector memory cell re-expressing CD45RA (EMRA) cells. Although the KRG group showed an increase in the number of CD4+ EMRA cells, the expression of senescent and exhausted markers in these cells decreased, and the capacity for senescence-related cytokine production in the senescent CD28- subset was ameliorated.
Conclusions: These findings suggest that KRG promotes the repopulation of CD4+ EMRA T cells and regulates phenotypical and functional senescent changes after ASCT in pediatric patients with cancer.
Keywords: Autologous hematopoietic stem cell transplantation; Childhood; Immunosenescence; Korea red ginseng; T cells.
© 2023 The Korean Society of Ginseng. Publishing services by Elsevier B.V.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures



Similar articles
-
Immune Reconstitution following High-Dose Chemotherapy and Autologous Stem Cell Transplantation with or without Pembrolizumab Maintenance Therapy in Patients with Lymphoma.Transplant Cell Ther. 2022 Jan;28(1):32.e1-32.e10. doi: 10.1016/j.jtct.2021.10.010. Epub 2021 Oct 17. Transplant Cell Ther. 2022. PMID: 34670169 Free PMC article. Clinical Trial.
-
Effect of Korean Red Ginseng intake on the survival duration of human immunodeficiency virus type 1 patients.J Ginseng Res. 2017 Apr;41(2):222-226. doi: 10.1016/j.jgr.2016.12.006. Epub 2017 Jan 6. J Ginseng Res. 2017. PMID: 28413328 Free PMC article.
-
The effect of red ginseng extract on inflammatory cytokines after chemotherapy in children.J Ginseng Res. 2012 Oct;36(4):383-90. doi: 10.5142/jgr.2012.36.4.383. J Ginseng Res. 2012. PMID: 23717140 Free PMC article.
-
Effect of Korean Red Ginseng on metabolic syndrome.J Ginseng Res. 2021 May;45(3):380-389. doi: 10.1016/j.jgr.2020.11.002. Epub 2020 Nov 12. J Ginseng Res. 2021. PMID: 34025131 Free PMC article. Review.
-
The beneficial potential of ginseng for menopause.J Ginseng Res. 2024 Sep;48(5):449-453. doi: 10.1016/j.jgr.2024.05.008. Epub 2024 May 31. J Ginseng Res. 2024. PMID: 39263310 Free PMC article. Review.
References
-
- Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. - PubMed
-
- Ness K.K., Kirkland J.L., Gramatges M.M., Wang Z., Kundu M., McCastlain K., Li-Harms X., Zhang J., Tchkonia T., Pluijm S.M.F., et al. Premature physiologic aging as a paradigm for understanding increased risk of adverse Health across the lifespan of survivors of childhood cancer. J Clin Oncol. 2018;36(21):2206–2215. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

