Repaglinide-Solid Lipid Nanoparticles in Chitosan Patches for Transdermal Application: Box-Behnken Design, Characterization, and In Vivo Evaluation
- PMID: 38223883
- PMCID: PMC10788056
- DOI: 10.2147/IJN.S438564
Repaglinide-Solid Lipid Nanoparticles in Chitosan Patches for Transdermal Application: Box-Behnken Design, Characterization, and In Vivo Evaluation
Abstract
Background: Repaglinide (REP) is an antidiabetic drug with limited oral bioavailability attributable to its low solubility and considerable first-pass hepatic breakdown. This study aimed to develop a biodegradable chitosan-based system loaded with REP-solid lipid nanoparticles (REP-SLNs) for controlled release and bioavailability enhancement via transdermal delivery.
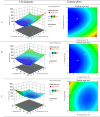
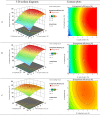
Methods: REP-SLNs were fabricated by ultrasonic hot-melt emulsification. A Box-Behnken design (BBD) was employed to explore and optimize the impacts of processing variables (lipid content, surfactant concentration, and sonication amplitude) on particle size (PS), and entrapment efficiency (EE). The optimized REP-SLN formulation was then incorporated within a chitosan solution to develop a transdermal delivery system (REP-SLN-TDDS) and evaluated for physicochemical properties, drug release, and ex vivo permeation profiles. Pharmacokinetic and pharmacodynamic characteristics were assessed using experimental rats.
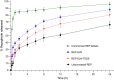
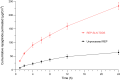
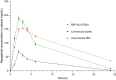
Results: The optimized REP-SLNs had a PS of 249±9.8 nm and EE of 78%±2.3%. The developed REP-SLN-TDDS demonstrated acceptable characteristics without significant aggregation of REP-SLNs throughout the casting and drying processes. The REP-SLN-TDDS exhibited a biphasic release pattern, where around 36% of the drug load was released during the first 2 h, then the drug release was sustained at around 80% at 24 h. The computed flux across rat skin for the REP-SLN-TDDS was 2.481±0.22 μg/cm2/h in comparison to 0.696±0.07 μg/cm2/h for the unprocessed REP, with an enhancement ratio of 3.56. The REP-SLN-TDDS was capable of sustaining greater REP plasma levels over a 24 h period (p<0.05). The REP-SLN-TDDS also reduced blood glucose levels compared to unprocessed REP and commercial tablets (p<0.05) in experimental rats.
Conclusion: Our REP-SLN-TDDS can be considered an efficient therapeutic option for REP administration.
Keywords: Box–Behnken design; bioavailability; chitosan; repaglinide; solid-lipid nanoparticles; transdermal.
© 2024 Ali et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures






References
-
- Vasam M, Maddiboyina B, Talluri C, Alagarsamy S, Gugulothu B, Roy H. Formulation, characterization, and Taguchi design study of eplerenone lipid-based solid dispersions integrated with gelucire. BioNanoScience. 2023;13(2):576–587. doi:10.1007/s12668-023-01102-4 - DOI
-
- Patravale VB, Mirani AG. Preparation and characterization of solid lipid nanoparticles-based gel for topical delivery. In: Weissig V, Elbayoumi T, editors. Pharmaceutical Nanotechnology: Basic Protocols. New York: Springer New York; 2019:293–302. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

