Strawberry Protease as a Laundry Detergent Additive Candidate: Immobilization, Compatibility Study with Detergent Ingredients, and Washing Performance Test
- PMID: 38223888
- PMCID: PMC10784196
- DOI: 10.1002/gch2.202300102
Strawberry Protease as a Laundry Detergent Additive Candidate: Immobilization, Compatibility Study with Detergent Ingredients, and Washing Performance Test
Abstract
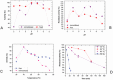
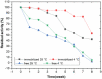
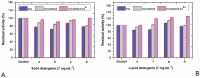
The potential of strawberry-derived protease as a component of laundry detergent is investigated. The compatibility of the enzyme with various surfactants, oxidizing agents, and commercial detergents is tested. The immobilized enzyme prepared by immobilizing Co2+ ions together with the enzyme is also tested. Strawberry crude protease shows high stability in the presence of surfactants frequently used in detergents. The enzyme is found to be relatively stable to oxidizing agents. In addition, it is determined that strawberry protease works in excellent compatibility with different commercial solid and liquid detergents in the Turkish market and also maintains its stability very well. Washing tests based on visual examination also reveal that the enzyme improves the washing performance of the tested detergent. All these properties and high activity at alkaline pH make this enzyme a very strong candidate for use in laundry detergent formulations.
Keywords: detergents; fragaria ananassa; immobilization; protease; strawberry.
© 2023 The Authors. Global Challenges published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Bacillus Swezeyi B2 Strain: A Novel Alkaliphilic Bacterium Producer of Alkaline-, Thermal, Oxidant-, and Surfactant-Stable Protease, Extremely Efficient in Detergency.Curr Microbiol. 2023 Feb 4;80(3):95. doi: 10.1007/s00284-022-03156-1. Curr Microbiol. 2023. PMID: 36737528
-
Laundry detergent compatibility of the alkaline protease from Bacillus cereus.Microbiol Res. 2004;159(2):135-40. doi: 10.1016/j.micres.2004.01.002. Microbiol Res. 2004. PMID: 15293947
-
Stability of thermostable alkaline protease from Bacillus licheniformis RP1 in commercial solid laundry detergent formulations.Microbiol Res. 2008;163(3):299-306. doi: 10.1016/j.micres.2006.06.001. Epub 2006 Jul 26. Microbiol Res. 2008. PMID: 16872818
-
Detergent-compatible proteases: microbial production, properties, and stain removal analysis.Prep Biochem Biotechnol. 2015;45(3):233-58. doi: 10.1080/10826068.2014.907183. Prep Biochem Biotechnol. 2015. PMID: 24678620 Review.
-
Laundry detergents: an overview.J Oleo Sci. 2007;56(7):327-40. doi: 10.5650/jos.56.327. J Oleo Sci. 2007. PMID: 17898499 Review.
Cited by
-
Biochemical characterization of immobilized recombinant subtilisin and synthesis and functional characterization of recombinant subtilisin capped silver and zinc oxide nanoparticles.Saudi J Biol Sci. 2024 Jul;31(7):104009. doi: 10.1016/j.sjbs.2024.104009. Epub 2024 May 4. Saudi J Biol Sci. 2024. PMID: 38766505 Free PMC article.
References
-
- Morellon‐Sterling R., El‐Siar H., Tavano O. L., Berenguer‐Murcia Á., Fernández‐Lafuente R., Int. J. Biol. Macromol. 2020, 162, 394. - PubMed
-
- Barzkar N., Int. J. Biol. Macromol. 2020, 161, 1216. - PubMed
-
- Gurumallesh P., Alagu K., Ramakrishnan B., Muthusamy S., Int. J. Biol. Macromol. 2019, 128, 254. - PubMed
-
- Barberis S. E., Quiroga E., Barcia C. S., Liggieri C., Electron J. Biotechnol. 2013, 16, 1.
LinkOut - more resources
Full Text Sources

