Impact of transcatheter heart valve type on outcomes of surgical explantation after failed transcatheter aortic valve replacement: the EXPLANT-TAVR international registry
- PMID: 38224255
- PMCID: PMC10786178
- DOI: 10.4244/EIJ-D-23-00722
Impact of transcatheter heart valve type on outcomes of surgical explantation after failed transcatheter aortic valve replacement: the EXPLANT-TAVR international registry
Abstract
Background: There are limited data on the impact of transcatheter heart valve (THV) type on the outcomes of surgical explantation after THV failure.
Aims: We sought to determine the outcomes of transcatheter aortic valve replacement (TAVR) explantation for failed balloon-expandable valves (BEV) versus self-expanding valves (SEV).
Methods: From November 2009 to February 2022, 401 patients across 42 centres in the EXPLANT-TAVR registry underwent TAVR explantation during a separate admission from the initial TAVR. Mechanically expandable valves (N=10, 2.5%) were excluded. The outcomes of TAVR explantation were compared for 202 (51.7%) failed BEV and 189 (48.3%) failed SEV.
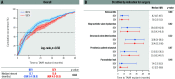
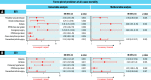
Results: Among 391 patients analysed (mean age: 73.0±9.8 years; 33.8% female), the median time from index TAVR to TAVR explantation was 13.3 months (interquartile range 5.1-34.8), with no differences between groups. Indications for TAVR explantation included endocarditis (36.0% failed SEV vs 55.4% failed BEV; p<0.001), paravalvular leak (21.2% vs 11.9%; p=0.014), structural valve deterioration (30.2% vs 21.8%; p=0.065) and prosthesis-patient mismatch (8.5% vs 10.4%; p=0.61). The SEV group trended fewer urgent/emergency surgeries (52.0% vs 62.3%; p=0.057) and more root replacement (15.3% vs 7.4%; p=0.016). Concomitant cardiac procedures were performed in 57.8% of patients, including coronary artery bypass graft (24.8%), and mitral (38.9%) and tricuspid (14.6%) valve surgery, with no differences between groups. In-hospital, 30-day, and 1-year mortality and stroke rates were similar between groups (allp>0.05), with no differences in cumulative mortality at 3 years (log-rank p=0.95). On multivariable analysis, concomitant mitral surgery was an independent predictor of 1-year mortality after BEV explant (hazard ratio [HR] 2.00, 95% confidence interval [CI]: 1.07-3.72) and SEV explant (HR 2.00, 95% CI: 1.08-3.69).
Conclusions: In the EXPLANT-TAVR global registry, BEV and SEV groups had different indications for surgical explantation, with more root replacements in SEV failure, but no differences in midterm mortality and morbidities. Further refinement of TAVR explantation techniques are important to improving outcomes.
Conflict of interest statement
G. Tang is a physician proctor and consultant for Medtronic; a consultant and physician advisory board member for Abbott Structural Heart; and a physician advisory board member for JenaValve. N.S. Kleiman has been involved in clinical trials for Edwards Lifesciences, Medtronic, and Boston Scientific; is involved in clinical education for Medtronic; and is on the steering committee for Boston Scientific. M. Szerlip is a physician proctor and consultant for Edwards Lifesciences; has received speaker honoraria for Boston Scientific; served as an advisory board member for Abbott; and is on the steering committee for Medtronic. M. Mack served as co-primary investigator for the PARTNER trial for Edwards Lifesciences and the COAPT trial for Abbott; and served as the study Chair for the APOLLO trial for Medtronic. T. Nazif has equity in Venus Medtech; and has received consulting fees or honoraria from Keystone Heart, Edwards Lifesciences, Medtronic, and Boston Scientific. A. Unbehaun has served as a physician proctor for Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic. M. Andreas is a physician proctor and consultant and has received speaker honoraria from Edwards Lifesciences, Abbott, and Medtronic; and has received institutional research grants from Edwards Lifesciences, Abbott, Medtronic, and LSI Solutions. D. Brinster is a consultant and speaker for CryoLife, Cook Medical, and Terumo Aortic. B. Ramlawi is a consultant for Boston Scientific, Medtronic, LivaNova, and AtriCure. L. Conradi is a physician proctor, consultant and speaker for Edwards Lifesciences, Medtronic, Abbott, and Boston Scientific. N. Desai reports institution research funding and speaker fees from Gore and Medtronic. J. Forrest is a physician proctor, consultant, and member of the advisory board for Edwards Lifesciences and Medtronic. T. Nguyen has received speaker honoraria from Edwards Lifesciences, CryoLife, and Abbott. R. Waksman is a consultant and advisory board member for Abbott Vascular, Amgen, Boston Scientific, Medtronic, Philips/Volcano, and Pi-Cardia; has received speaker honoraria and grant support from AstraZeneca and Chiesi; has received grant support from Biotronik; is a consultant for Transmural Solutions; and is an investor for MedAlliance and Transmural Solutions. L. Leroux is a physician proctor for Medtronic and Abbott; and a consultant for Edwards Lifesciences. K. Grubb is a physician proctor for Medtronic, Edwards Lifesciences, and Boston Scientific; and has served as a consultant for Medtronic, Boston Scientific, Ancora, HLT, and BioVentrix. P. Denti receives speaker honoraria from Abbott and Edwards Lifesciences; and is a consultant for InnovHeart. T. Modine is a physician proctor and consultant for Medtronic, Edwards Lifesciences, and Abbott. V. Bapat has served as a consultant for Medtronic, Edwards Lifesciences, 4C Medical, and Boston Scientific. T. Kaneko is a speaker for Edwards Lifesciences, Medtronic, Abbott, and Baylis Medical; and is a consultant for 4C Medical. M. Reardon is a consultant for Medtronic, Boston Scientific, Abbott, and W.L. Gore & Associates. G. Bruschi is a proctor and consultant for Medtronic and Abbott. P.B. Shah is a proctor and consultant for Edwards Lifesciences; is an advisory board member for Xenter; and has received educational grants from Medtronic, Abbott, and Edwards Lifesciences. N. M. Van Mieghem has received research grant support from Abbott, Boston Scientific, Medtronic, Edwards Lifesciences, Daiichi Sankyo, and PulseCath BV. J. Kempfert has served as a proctor for Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic. R. Lange is a consultant for Medtronic. D. Atkins has received speaker honoraria from Medtronic. B. K. Whisenant is a consultant for Edwards Lifesciences. M. A. Borger discloses that his hospital receives speaker honoraria and/or consulting fees on his behalf from Edwards Lifesciences, Medtronic, Abbott, and CryoLife. L. Pirelli is a proctor for and has received speaker honoraria from Edwards Lifesciences; and is a consultant for Medtronic. C. Hagl has received speaker honoraria from Edwards Lifesciences. M. W. A. Chu has received speaker honoraria from Medtronic, Edwards Lifesciences, and Terumo Aortic. M. H. Salinger has served as a consultant for Boston Scientific and Edwards Lifesciences. C. Shults is a physician proctor, consultant and has received speaker honoraria for AtriCure and Terumo Aortic. W. Ben Ali has received research grants from Edwards Lifesciences and Medtronic. R. Ibrahim is a proctor for Abbott and Boston Scientific, and a consultant for Abbott, Boston Scientific, Medtronic, and Occlutech. J. Rodés-Cabau has received institutional research grants from and is a consultant for Edwards Lifesciences, Medtronic, and Boston Scientific. F. Maisano has received grant and/or research support from Abbott, Medtronic, Edwards Lifesciences, Biotronik, Boston Scientific, New Valve Technology, and Terumo; and has received consulting fees and honoraria from Abbott, Medtronic, Edwards Lifesciences, and SwissVortex. P. M. Ouzounian serves as a consultant for Medtronic and is on their North American advisory board. O. D. Bhadra has received travel compensation from Edwards Lifesciences. R. Estevez-Loureiro has received speaker honoraria and is a proctor for Abbott, Edwards Lifesciences, and Boston Scientific. M. Wyler von Ballmoos has served as a consultant for LivaNova, Medtronic, and Boston Scientific. S. Fukuhara is a consultant for Terumo Aortic. G. M. Deeb is on the Surgical Advisory Board for Medtronic and a member of the Executive, Screening and Steering Committees for the Medtronic FDA TAVR Trials. T. Al-Atassi has served on the advisory board for Edwards Lifesciences, Medtronic, and Abbott; and he has proctored for Edwards Lifesciences. J. E. Bavaria is a consultant for Edwards Lifesciences and Medtronic; and has served as a clinical trial principal investigator for Edwards Lifesciences, Medtronic, and Abbott; he has also served as the Chairman of the Data Safety Monitoring Board for Abbott. A. Geirsson is a member of Medtronic Strategic Surgical Advisory Board. M. Taramasso has been a consultant for Abbott, Boston Scientific, Edwards Lifesciences, 4tech, Mitraltech, Simulands, MTEx, Occlufit, CoreMedic, and Shenqi Medical. M. Gennari is a consultant for Medtronic. The other authors/collaborators have no conflicts of interest to declare.
Figures


References
-
- Unbehaun A, Abdullah M, Hooda A, Gedela M, Kempfert J, Klein C, Tang GHL. TAVR - From inoperable to younger, lower-risk patients: A slippery slope? Prog Cardiovasc Dis. 2022;72:41–53. - PubMed
-
- Carroll JD, Mack MJ, Vemulapalli S, Herrmann HC, Gleason TG, Hanzel G, Deeb GM, Thourani VH, Cohen DJ, Desai N, Kirtane AJ, Fitzgerald S, Michaels J, Krohn C, Masoudi FA, Brindis RG, Bavaria JE. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2020;76:2492–516. - PubMed
-
- Tang GHL, Zaid S, Gupta E, Ahmad H, Khan A, Kovacic JC, Lansman SL, Dangas GD, Sharma SK, Kini A. Feasibility of Repeat TAVR After SAPIEN 3 TAVR: A Novel Classification Scheme and Pilot Angiographic Study. JACC Cardiovasc Interv. 2019;12:1290–2. - PubMed
-
- Tang GHL, Zaid S, Kleiman NS, Goel SS, Fukuhara S, Marin-Cuartas M, Kiefer P, Abdel-Wahab M, De Backer, Søndergaard L, Saha S, Hagl C, Wyler von, Bhadra O, Conradi L, Grubb KJ, Shih E, DiMaio JM, Szerlip M, Vitanova K, Ruge H, Unbehaun A, Kempfert J, Pirelli L, Kliger CA, Van Mieghem, Hokken TW, Adrichem R, Modine T, Corona S, Wang L, Petrossian G, Robinson N, Meier D, Webb JG, Cheung A, Ramlawi B, Herrmann HC, Desai ND, Andreas M, Mach M, Waksman R, Schults CC, Ahmad H, Goldberg JB, Geirsson A, Forrest JK, Denti P, Belluschi I, Ben-Ali W, Asgar AW, Taramasso M, Rovin JD, Di Eusanio, Colli A, Kaneko T, Nazif TN, Leon MB, Bapat VN, Mack MJ, Reardon MJ, Sathananthan J. Explant vs Redo-TAVR After Transcatheter Valve Failure: Mid-Term Outcomes From the EXPLANTORREDO-TAVR International Registry. JACC Cardiovasc Interv. 2023;16:927–41. - PubMed
-
- Rogers T, Greenspun BC, Weissman G, Torguson R, Craig P, Shults C, Gordon P, Ehsan A, Wilson SR, Goncalves J, Levitt R, Hahn C, Parikh P, Bilfinger T, Butzel D, Buchanan S, Hanna N, Garrett R, Buchbinder M, Asch F, Garcia-Garcia HM, Okubagzi P, Ben-Dor I, Satler LF, Waksman R. Feasibility of Coronary Access and Aortic Valve Reintervention in Low-Risk TAVR Patients. JACC Cardiovasc Interv. 2020;13:726–35. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
