Effects of COVID-19 mRNA vaccination on HIV viremia and reservoir size
- PMID: 38224350
- PMCID: PMC11139238
- DOI: 10.1097/QAD.0000000000003841
Effects of COVID-19 mRNA vaccination on HIV viremia and reservoir size
Abstract
Objective: The immunogenic nature of coronavirus disease 2019 (COVID-19) mRNA vaccines led to some initial concern that these could stimulate the HIV reservoir. We analyzed changes in plasma HIV loads (pVL) and reservoir size following COVID-19 mRNA vaccination in 62 people with HIV (PWH) receiving antiretroviral therapy (ART), and analyzed province-wide trends in pVL before and after the mass vaccination campaign.
Design: Longitudinal observational cohort and province-wide analysis.
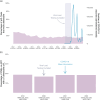
Methods: Sixty-two participants were sampled prevaccination, and one month after their first and second COVID-19 immunizations. Vaccine-induced anti-SARS-CoV-2-Spike antibodies in serum were measured using the Roche Elecsys Anti-S assay. HIV reservoirs were quantified using the intact proviral DNA assay; pVL were measured using the cobas 6800 (lower limit of quantification: 20 copies/ml). The province-wide analysis included all 290 401 pVL performed in British Columbia, Canada between 2012 and 2022.
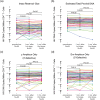
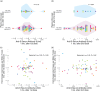
Results: Prevaccination, the median intact reservoir size was 77 [interquartile range (IQR): 20-204] HIV copies/million CD4 + T-cells, compared to 74 (IQR: 27-212) and 65 (IQR: 22-174) postfirst and -second dose, respectively (all comparisons P > 0.07). Prevaccination, 82% of participants had pVL <20 copies/ml (max: 110 copies/ml), compared to 79% postfirst dose (max: 183 copies/ml) and 85% postsecond dose (max: 79 copies/ml) ( P > 0.4). There was no evidence that the magnitude of the vaccine-elicited anti-SARS-CoV-2-Spike immune response influenced pVL nor changes in reservoir size ( P > 0.6). We found no evidence linking the COVID-19 mass vaccination campaign to population-level increases in detectable pVL frequency among all PWH in the province, nor among those who maintained pVL suppression on ART.
Conclusion: We found no evidence that COVID-19 mRNA vaccines induced changes in HIV reservoir size nor plasma viremia.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

Update of
-
Effects of COVID-19 mRNA vaccination on HIV viremia and reservoir size.medRxiv [Preprint]. 2023 Oct 9:2023.10.08.23296718. doi: 10.1101/2023.10.08.23296718. medRxiv. 2023. Update in: AIDS. 2024 Jul 1;38(8):1120-1130. doi: 10.1097/QAD.0000000000003841. PMID: 37873490 Free PMC article. Updated. Preprint.
References
-
- Geretti AM, Stockdale AJ, Kelly SH, Cevik M, Collins S, Waters L, et al. Outcomes of coronavirus disease 2019 (COVID-19) related hospitalization among people with human immunodeficiency virus (HIV) in the ISARIC World Health Organization (WHO) Clinical Characterization Protocol (UK): a prospective observational study. Clin Infect Dis 2020; 73:e2095–e2106. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

