Chemoprevention for malaria with monthly intermittent preventive treatment with dihydroartemisinin-piperaquine in pregnant women living with HIV on daily co-trimoxazole in Kenya and Malawi: a randomised, double-blind, placebo-controlled trial
- PMID: 38224710
- PMCID: PMC10865779
- DOI: 10.1016/S0140-6736(23)02631-4
Chemoprevention for malaria with monthly intermittent preventive treatment with dihydroartemisinin-piperaquine in pregnant women living with HIV on daily co-trimoxazole in Kenya and Malawi: a randomised, double-blind, placebo-controlled trial
Abstract
Background: The efficacy of daily co-trimoxazole, an antifolate used for malaria chemoprevention in pregnant women living with HIV, is threatened by cross-resistance of Plasmodium falciparum to the antifolate sulfadoxine-pyrimethamine. We assessed whether addition of monthly dihydroartemisinin-piperaquine to daily co-trimoxazole is more effective at preventing malaria infection than monthly placebo plus daily co-trimoxazole in pregnant women living with HIV.
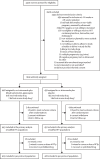
Methods: We did an individually randomised, two-arm, placebo-controlled trial in areas with high-grade sulfadoxine-pyrimethamine resistance in Kenya and Malawi. Pregnant women living with HIV on dolutegravir-based combination antiretroviral therapy (cART) who had singleton pregnancies between 16 weeks' and 28 weeks' gestation were randomly assigned (1:1) by computer-generated block randomisation, stratified by site and HIV status (known positive vs newly diagnosed), to daily co-trimoxazole plus monthly dihydroartemisinin-piperaquine (three tablets of 40 mg dihydroartemisinin and 320 mg piperaquine given daily for 3 days) or daily co-trimoxazole plus monthly placebo. Daily co-trimoxazole consisted of one tablet of 160 mg sulfamethoxazole and 800 mg trimethoprim. The primary endpoint was the incidence of Plasmodium infection detected in the peripheral (maternal) or placental (maternal) blood or tissue by PCR, microscopy, rapid diagnostic test, or placental histology (active infection) from 2 weeks after the first dose of dihydroartemisinin-piperaquine or placebo to delivery. Log-binomial regression was used for binary outcomes, and Poisson regression for count outcomes. The primary analysis was by modified intention to treat, consisting of all randomised eligible participants with primary endpoint data. The safety analysis included all women who received at least one dose of study drug. All investigators, laboratory staff, data analysts, and participants were masked to treatment assignment. This trial is registered with ClinicalTrials.gov, NCT04158713.
Findings: From Nov 11, 2019, to Aug 3, 2021, 904 women were enrolled and randomly assigned to co-trimoxazole plus dihydroartemisinin-piperaquine (n=448) or co-trimoxazole plus placebo (n=456), of whom 895 (99%) contributed to the primary analysis (co-trimoxazole plus dihydroartemisinin-piperaquine, n=443; co-trimoxazole plus placebo, n=452). The cumulative risk of any malaria infection during pregnancy or delivery was lower in the co-trimoxazole plus dihydroartemisinin-piperaquine group than in the co-trimoxazole plus placebo group (31 [7%] of 443 women vs 70 [15%] of 452 women, risk ratio 0·45, 95% CI 0·30-0·67; p=0·0001). The incidence of any malaria infection during pregnancy or delivery was 25·4 per 100 person-years in the co-trimoxazole plus dihydroartemisinin-piperaquine group versus 77·3 per 100 person-years in the co-trimoxazole plus placebo group (incidence rate ratio 0·32, 95% CI 0·22-0·47, p<0·0001). The number needed to treat to avert one malaria infection per pregnancy was 7 (95% CI 5-10). The incidence of serious adverse events was similar between groups in mothers (17·7 per 100 person-years in the co-trimoxazole plus dihydroartemisinin-piperaquine group [23 events] vs 17·8 per 100 person-years in the co-trimoxazole group [25 events]) and infants (45·4 per 100 person-years [23 events] vs 40·2 per 100 person-years [21 events]). Nausea within the first 4 days after the start of treatment was reported by 29 (7%) of 446 women in the co-trimoxazole plus dihydroartemisinin-piperaquine group versus 12 (3%) of 445 women in the co-trimoxazole plus placebo group. The risk of adverse pregnancy outcomes did not differ between groups.
Interpretation: Addition of monthly intermittent preventive treatment with dihydroartemisinin-piperaquine to the standard of care with daily unsupervised co-trimoxazole in areas of high antifolate resistance substantially improves malaria chemoprevention in pregnant women living with HIV on dolutegravir-based cART and should be considered for policy.
Funding: European and Developing Countries Clinical Trials Partnership 2; UK Joint Global Health Trials Scheme (UK Foreign, Commonwealth and Development Office; Medical Research Council; National Institute for Health Research; Wellcome); and Swedish International Development Cooperation Agency.
Copyright © 2024 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures


Comment in
-
Dihydroartemisinin-piperaquine for prevention of malaria in pregnant women living with HIV.Lancet. 2024 Jan 27;403(10424):327-330. doi: 10.1016/S0140-6736(24)00048-5. Epub 2024 Jan 12. Lancet. 2024. PMID: 38224711 No abstract available.
References
-
- ter Kuile FO, Parise ME, Verhoeff FH, et al. The burden of co-infection with human immunodeficiency virus type 1 and malaria in pregnant women in sub-saharan Africa. Am J Trop Med Hyg. 2004;71:41–54. - PubMed
-
- Desai M, Hill J, Fernandes S, et al. Prevention of malaria in pregnancy. Lancet Infect Dis. 2018;18:e119–e132. - PubMed
-
- Denoeud-Ndam L, Zannou D-M, Fourcade C, et al. Cotrimoxazole prophylaxis versus mefloquine intermittent preventive treatment to prevent malaria in HIV-infected pregnant women: two randomized controlled trials. J Acquir Immune Defic Syndr. 2014;65:198–206. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

