The Basis for Weekly Insulin Therapy: Evolving Evidence With Insulin Icodec and Insulin Efsitora Alfa
- PMID: 38224978
- PMCID: PMC11091825
- DOI: 10.1210/endrev/bnad037
The Basis for Weekly Insulin Therapy: Evolving Evidence With Insulin Icodec and Insulin Efsitora Alfa
Erratum in
-
Correction to: "The Basis for Weekly Insulin Therapy: Evolving Evidence With Insulin Icodec and Insulin Efsitora Alfa".Endocr Rev. 2024 May 7;45(3):436. doi: 10.1210/endrev/bnae012. Endocr Rev. 2024. PMID: 38518751 Free PMC article. No abstract available.
Abstract
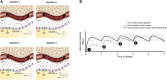
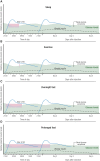
Basal insulin continues to be a vital part of therapy for many people with diabetes. First attempts to prolong the duration of insulin formulations were through the development of suspensions that required homogenization prior to injection. These insulins, which required once- or twice-daily injections, introduced wide variations in insulin exposure contributing to unpredictable effects on glycemia. Advances over the last 2 decades have resulted in long-acting, soluble basal insulin analogues with prolonged and less variable pharmacokinetic exposure, improving their efficacy and safety, notably by reducing nocturnal hypoglycemia. However, adherence and persistence with once-daily basal insulin treatment remains low for many reasons including hypoglycemia concerns and treatment burden. A soluble basal insulin with a longer and flatter exposure profile could reduce pharmacodynamic variability, potentially reducing hypoglycemia, have similar efficacy to once-daily basal insulins, simplify dosing regimens, and improve treatment adherence. Insulin icodec (Novo Nordisk) and insulin efsitora alfa (basal insulin Fc [BIF], Eli Lilly and Company) are 2 such insulins designed for once-weekly administration, which have the potential to provide a further advance in basal insulin replacement. Icodec and efsitora phase 2 clinical trials, as well as data from the phase 3 icodec program indicate that once-weekly insulins provide comparable glycemic control to once-daily analogues, with a similar risk of hypoglycemia. This manuscript details the technology used in the development of once-weekly basal insulins. It highlights the clinical rationale and potential benefits of these weekly insulins while also discussing the limitations and challenges these molecules could pose in clinical practice.
Keywords: basal insulin; hypoglycemia; insulin efsitora; insulin icodec; once-weekly insulin; peak-to-trough ratio.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Endocrine Society.
Figures











References
-
- Beals JM, DeFelippis MR, Paavola CD, Allen DP, Garg A. Bruce Baldwin D. Insulin. In: Crommelin DJA Sindelar RD and Meibohm B (eds.), Pharmaceutical Biotechnology: Fundamentals and Applications. Springer International Publishing; 2019:403‐427.
-
- Heinemann L, Linkeschova R, Rave K, Hompesch B, Sedlak M, Heise T. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care. 2000;23(5):644‐649. - PubMed
-
- Riddle MC, Rosenstock J, Gerich J; Insulin Glargine 4002 Study Investigators . The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26(11):3080‐3086 - PubMed
-
- Yki-Järvinen H, Dressler A, Ziemen M; HOE 901/3002 Study Group . Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care. 2000;23(8):1130‐1136 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

