Tolerable glycometabolic stress boosts cancer cell resilience through altered N-glycosylation and Notch signaling activation
- PMID: 38225221
- PMCID: PMC10789756
- DOI: 10.1038/s41419-024-06432-z
Tolerable glycometabolic stress boosts cancer cell resilience through altered N-glycosylation and Notch signaling activation
Abstract
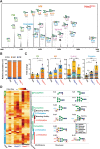
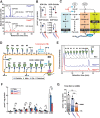
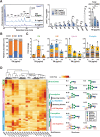
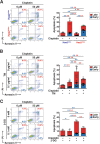
Chronic metabolic stress paradoxically elicits pro-tumorigenic signals that facilitate cancer stem cell (CSC) development. Therefore, elucidating the metabolic sensing and signaling mechanisms governing cancer cell stemness can provide insights into ameliorating cancer relapse and therapeutic resistance. Here, we provide convincing evidence that chronic metabolic stress triggered by hyaluronan production augments CSC-like traits and chemoresistance by partially impairing nucleotide sugar metabolism, dolichol lipid-linked oligosaccharide (LLO) biosynthesis and N-glycan assembly. Notably, preconditioning with either low-dose tunicamycin or 2-deoxy-D-glucose, which partially interferes with LLO biosynthesis, reproduced the promoting effects of hyaluronan production on CSCs. Multi-omics revealed characteristic changes in N-glycan profiles and Notch signaling activation in cancer cells exposed to mild glycometabolic stress. Restoration of N-glycan assembly with glucosamine and mannose supplementation and Notch signaling blockade attenuated CSC-like properties and further enhanced the therapeutic efficacy of cisplatin. Therefore, our findings uncover a novel mechanism by which tolerable glycometabolic stress boosts cancer cell resilience through altered N-glycosylation and Notch signaling activation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

