Synthesis of inter-[60]fullerene conjugates with inherent chirality
- PMID: 38225251
- PMCID: PMC10789730
- DOI: 10.1038/s41467-024-44834-x
Synthesis of inter-[60]fullerene conjugates with inherent chirality
Abstract
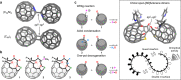
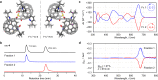
Coalescence of [60]fullerenes potentially produces hypothetical nanocarbon assemblies with non-naturally occurring topologies. Since the discovery of [60]fullerene in 1985, coalesced [60]fullerene oligomers have only been observed as transient species by transmission electron microscopy during an oligomerization process under a high electron acceleration voltage. Herein, we showcase the rational synthesis of covalent assemblies consisting of inherently chiral open-[60]fullerenes. The crystallographic analyses unveiled double-caged structures of non-conjugated and conjugated inter-[60]fullerene hybrids, in which the two [60]fullerene cages are bounds to each other through a covalent linkage. The former one further assembles via a heterochiral recognition so that four carbon cages are arranged in a tetrahedral manner both in solution and solid state. Reflecting radially-conjugated double π-surface nature, the inter-[60]fullerene conjugate exhibits strong electronic communication in its reduced states, intense absorption behavior, and chiroptical activity with a dissymmetry factor of 0.21 (at 674 nm) which breaks the record for known chiral organic molecules.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Near-Infrared-Absorbing Chiral Open [60]Fullerenes.Angew Chem Int Ed Engl. 2023 Jan 9;62(2):e202215380. doi: 10.1002/anie.202215380. Epub 2022 Dec 2. Angew Chem Int Ed Engl. 2023. PMID: 36357327
-
Fullerenes as Nanocontainers That Stabilize Unique Actinide Species Inside: Structures, Formation, and Reactivity.Acc Chem Res. 2019 Jul 16;52(7):1824-1833. doi: 10.1021/acs.accounts.9b00229. Epub 2019 Jul 1. Acc Chem Res. 2019. PMID: 31260256
-
Chiroptical Response of Carbon Nanocages Enhanced by Achiral Guests.Angew Chem Int Ed Engl. 2025 Mar 10;64(11):e202421859. doi: 10.1002/anie.202421859. Epub 2024 Dec 4. Angew Chem Int Ed Engl. 2025. PMID: 39603987
-
The stabilization of fused-pentagon fullerene molecules.Nat Chem. 2009 Sep;1(6):450-60. doi: 10.1038/nchem.329. Epub 2009 Aug 24. Nat Chem. 2009. PMID: 21378913 Review.
-
Fullerene-biomolecule conjugates and their biomedicinal applications.Int J Nanomedicine. 2014;9:77-92. doi: 10.2147/IJN.S52829. Epub 2013 Dec 18. Int J Nanomedicine. 2014. PMID: 24379667 Free PMC article. Review.
Cited by
-
Twisted Nanographenes with Robust Conformational Stability.Nanomaterials (Basel). 2024 Oct 30;14(21):1737. doi: 10.3390/nano14211737. Nanomaterials (Basel). 2024. PMID: 39513817 Free PMC article.
-
Raman Spectroscopy of Fullerenes: From C60 to Functionalized Derivatives.Molecules. 2025 Feb 6;30(3):738. doi: 10.3390/molecules30030738. Molecules. 2025. PMID: 39942840 Free PMC article. Review.
-
Hydrophilization and Functionalization of Fullerene C60 with Maleic Acid Copolymers by Forming a Non-Covalent Complex.Polymers (Basel). 2024 Jun 19;16(12):1736. doi: 10.3390/polym16121736. Polymers (Basel). 2024. PMID: 38932086 Free PMC article.
-
Chiral Pd2L4 Capsules from Readily Accessible Tröger's Base Ligands Inducing Circular Dichroism on Fullerenes C60 and C70.Angew Chem Int Ed Engl. 2025 Mar 3;64(10):e202421137. doi: 10.1002/anie.202421137. Epub 2024 Dec 12. Angew Chem Int Ed Engl. 2025. PMID: 39625997 Free PMC article.
-
Nanoarchitectonics for Pentagon Defects in Carbon: Properties and Catalytic Role in Oxygen Reduction Reaction.Small Methods. 2025 Aug;9(8):e2500069. doi: 10.1002/smtd.202500069. Epub 2025 Apr 22. Small Methods. 2025. PMID: 40263926 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

