Corynoxine promotes TFEB/TFE3-mediated autophagy and alleviates Aβ pathology in Alzheimer's disease models
- PMID: 38225393
- PMCID: PMC11053156
- DOI: 10.1038/s41401-023-01197-1
Corynoxine promotes TFEB/TFE3-mediated autophagy and alleviates Aβ pathology in Alzheimer's disease models
Abstract
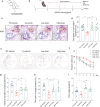
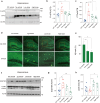
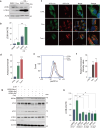
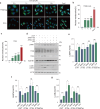
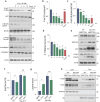
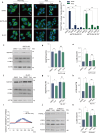
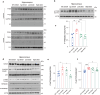
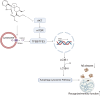
Autophagy impairment is a key factor in Alzheimer's disease (AD) pathogenesis. TFEB (transcription factor EB) and TFE3 (transcription factor binding to IGHM enhancer 3) are nuclear transcription factors that regulate autophagy and lysosomal biogenesis. We previously showed that corynoxine (Cory), a Chinese medicine compound, protects neurons from Parkinson's disease (PD) by activating autophagy. In this study, we investigated the effect of Cory on AD models in vivo and in vitro. We found that Cory improved learning and memory function, increased neuronal autophagy and lysosomal biogenesis, and reduced pathogenic APP-CTFs levels in 5xFAD mice model. Cory activated TFEB/TFE3 by inhibiting AKT/mTOR signaling and stimulating lysosomal calcium release via transient receptor potential mucolipin 1 (TRPML1). Moreover, we demonstrated that TFEB/TFE3 knockdown abolished Cory-induced APP-CTFs degradation in N2aSwedAPP cells. Our findings suggest that Cory promotes TFEB/TFE3-mediated autophagy and alleviates Aβ pathology in AD models.
Keywords: Alzheimer’s disease; TFEB/TFE3; autophagy; calcium; corynoxine.
© 2023. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

