In situ architecture of Opa1-dependent mitochondrial cristae remodeling
- PMID: 38225406
- PMCID: PMC10897290
- DOI: 10.1038/s44318-024-00027-2
In situ architecture of Opa1-dependent mitochondrial cristae remodeling
Abstract
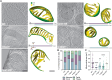
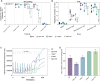
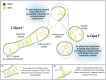
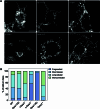
Cristae membrane state plays a central role in regulating mitochondrial function and cellular metabolism. The protein Optic atrophy 1 (Opa1) is an important crista remodeler that exists as two forms in the mitochondrion, a membrane-anchored long form (l-Opa1) and a processed short form (s-Opa1). The mechanisms for how Opa1 influences cristae shape have remained unclear due to lack of native three-dimensional views of cristae. We perform in situ cryo-electron tomography of cryo-focused ion beam milled mouse embryonic fibroblasts with defined Opa1 states to understand how each form of Opa1 influences cristae architecture. In our tomograms, we observe a variety of cristae shapes with distinct trends dependent on s-Opa1:l-Opa1 balance. Increased l-Opa1 levels promote cristae stacking and elongated mitochondria, while increased s-Opa1 levels correlated with irregular cristae packing and round mitochondria shape. Functional assays indicate a role for l-Opa1 in wild-type apoptotic and calcium handling responses, and show a compromised respiratory function under Opa1 imbalance. In summary, we provide three-dimensional visualization of cristae architecture to reveal relationships between mitochondrial ultrastructure and cellular function dependent on Opa1-mediated membrane remodeling.
Keywords: Cristae Remodeling; Cryo-Electron Tomography; Cryo-Focused Ion Beam Milling; Mitochondrial Biology.
© 2024. The Author(s).
Figures







Update of
-
In situ architecture of Opa1-dependent mitochondrial cristae remodeling.bioRxiv [Preprint]. 2023 Nov 9:2023.01.16.524176. doi: 10.1101/2023.01.16.524176. bioRxiv. 2023. Update in: EMBO J. 2024 Feb;43(3):391-413. doi: 10.1038/s44318-024-00027-2. PMID: 36711707 Free PMC article. Updated. Preprint.
References
-
- Bernardi P, Gerle C, Halestrap AP, Jonas EA, Karch J, Mnatsakanyan N, Pavlov E, Sheu S-S, Soukas AA. Identity, structure, and function of the mitochondrial permeability transition pore: controversies, consensus, recent advances, and future directions. Cell Death Differ. 2023;30:1869–1885. doi: 10.1038/s41418-023-01187-0. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

