Don't be late! Postponing cognitive decline and preventing early unemployment in people with multiple sclerosis: a study protocol
- PMID: 38225561
- PMCID: PMC10789039
- DOI: 10.1186/s12883-023-03513-y
Don't be late! Postponing cognitive decline and preventing early unemployment in people with multiple sclerosis: a study protocol
Abstract
Background: Up to 65% of people with multiple sclerosis (PwMS) develop cognitive deficits, which hampers their ability to work, participating in day-to-day life and ultimately reducing quality of life (QoL). Early cognitive symptoms are often less tangible to PwMS and their direct environment and are noticed only when symptoms and work functioning problems become more advanced, i.e., when (brain) damage is already advanced. Treatment of symptoms at a late stage can lead to cognitive impairment and unemployment, highlighting the need for preventative interventions in PwMS.
Aims: This study aims to evaluate the (cost-) effectiveness of two innovative preventative interventions, aimed at postponing cognitive decline and work functioning problems, compared to enhanced usual care in improving health-related QoL (HRQoL).
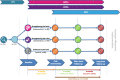
Methods: Randomised controlled trial including 270 PwMS with mild cognitive impairment, who have paid employment ≥ 12 h per week and are able to participate in physical exercise (Expanded Disability Status Scale < 6.0). Participants are randomised across three study arms: 1) 'strengthening the brain' - a lifestyle intervention combining personal fitness, mental coaching, dietary advice, and cognitive training; 2) 'strengthening the mind' - a work-focused intervention combining the capability approach and the participatory approach in one-on-one coaching by trained work coaches who have MS themselves; 3) Control group-receiving general information about cognitive impairment in MS and receiving care as usual. Intervention duration is four months, with short-term and long-term follow-up measurements at 10 and 16 months, respectively. The primary outcome measure of the Don't be late! intervention study will be HRQoL as measured with the 36-item Short Form. Secondary outcomes include cognition, work related outcomes, physical functioning, structural and functional brain changes, psychological functioning, and societal costs. Semi-structured interviews and focus groups with stakeholders will be organised to qualitatively reflect on the process and outcome of the interventions.
Discussion: This study seeks to prevent (further) cognitive decline and job loss due to MS by introducing tailor-made interventions at an early stage of cognitive symptoms, thereby maintaining or improving HRQoL. Qualitative analyses will be performed to allow successful implementation into clinical practice.
Trial registration: Retrospectively registered at ClinicalTrials.gov with reference number NCT06068582 on 10 October 2023.
Keywords: Cognition; Employment; Exercise; Health-related quality of life; Multiple sclerosis; Prevention.
© 2024. The Author(s).
Conflict of interest statement
JA, SS, JB, VG, BJ, MK, MR, FS, ES, SV, PW, GW, and KH have nothing to declare with respect to this study.
BU has received research support and/or consultancy fees from Biogen Idec, Genzyme, Merck Serono, Novartis, Roche, Teva and Immunic Therapeutics.
MS serves on the editorial board of Neurology, Multiple Sclerosis Journal and Frontiers in Neurology, receives research support from the Dutch MS Research Foundation, Eurostars-EUREKA, ARSEP, Amsterdam Neuroscience, MAGNIMS and ZonMW (Vidi grant, project number 09150172010056) and has served as a consultant for or received research support from Atara Biotherapeutics, Biogen, Celgene/Bristol Meyers Squibb, EIP, Sanofi, MedDay and Merck.
HH is an editor of the Multiple Sclerosis Journal controversies sections, receives research support from the Dutch MS Research Foundation and the Dutch Research Council. She has served as a consultant for or received research support from Atara Biotherapeutics, Biogen, Novartis, Celgene/Bristol Meyers Squibb, Sanofi Genzyme, MedDay and Merck BV.
Figures
References
Publication types
MeSH terms
Associated data
Grants and funding
- NWA.1292.19.064/Nederlandse Organisatie voor Wetenschappelijk Onderzoek
- NWA.1292.19.064/Nederlandse Organisatie voor Wetenschappelijk Onderzoek
- NWA.1292.19.064/Nederlandse Organisatie voor Wetenschappelijk Onderzoek
- NWA.1292.19.064/Nederlandse Organisatie voor Wetenschappelijk Onderzoek
- NWA.1292.19.064/Nederlandse Organisatie voor Wetenschappelijk Onderzoek
- NWA.1292.19.064/Nederlandse Organisatie voor Wetenschappelijk Onderzoek
- NWA.1292.19.064/Nederlandse Organisatie voor Wetenschappelijk Onderzoek
- NWA.1292.19.064/Nederlandse Organisatie voor Wetenschappelijk Onderzoek
- NWA.1292.19.064/Nederlandse Organisatie voor Wetenschappelijk Onderzoek
- NWA.1292.19.064/Nederlandse Organisatie voor Wetenschappelijk Onderzoek
- NWA.1292.19.064/Nederlandse Organisatie voor Wetenschappelijk Onderzoek
- NWA.1292.19.064/Nederlandse Organisatie voor Wetenschappelijk Onderzoek
- NWA.1292.19.064/Nederlandse Organisatie voor Wetenschappelijk Onderzoek
- NWA.1292.19.064/Nederlandse Organisatie voor Wetenschappelijk Onderzoek
- NWA.1292.19.064/Nederlandse Organisatie voor Wetenschappelijk Onderzoek
- NWA.1292.19.064/Nederlandse Organisatie voor Wetenschappelijk Onderzoek
LinkOut - more resources
Full Text Sources
Medical