Clinical antiviral efficacy of favipiravir in early COVID-19 (PLATCOV): an open-label, randomised, controlled, adaptive platform trial
- PMID: 38225598
- PMCID: PMC10789040
- DOI: 10.1186/s12879-023-08835-3
Clinical antiviral efficacy of favipiravir in early COVID-19 (PLATCOV): an open-label, randomised, controlled, adaptive platform trial
Abstract
In early symptomatic COVID-19 treatment, high dose oral favipiravir did not accelerate viral clearance.
Background: Favipiravir, an anti-influenza drug, has in vitro antiviral activity against SARS-CoV-2. Clinical trial evidence to date is inconclusive. Favipiravir has been recommended for the treatment of COVID-19 in some countries.
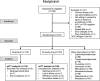
Methods: In a multicentre open-label, randomised, controlled, adaptive platform trial, low-risk adult patients with early symptomatic COVID-19 were randomised to one of ten treatment arms including high dose oral favipiravir (3.6g on day 0 followed by 1.6g daily to complete 7 days treatment) or no study drug. The primary outcome was the rate of viral clearance (derived under a linear mixed-effects model from the daily log10 viral densities in standardised duplicate oropharyngeal swab eluates taken daily over 8 days [18 swabs per patient]), assessed in a modified intention-to-treat population (mITT). The safety population included all patients who received at least one dose of the allocated intervention. This ongoing adaptive platform trial was registered at ClinicalTrials.gov (NCT05041907) on 13/09/2021.
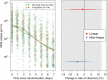
Results: In the final analysis, the mITT population contained data from 114 patients randomised to favipiravir and 126 patients randomised concurrently to no study drug. Under the linear mixed-effects model fitted to all oropharyngeal viral density estimates in the first 8 days from randomisation (4,318 swabs), there was no difference in the rate of viral clearance between patients given favipiravir and patients receiving no study drug; a -1% (95% credible interval: -14 to 14%) difference. High dose favipiravir was well-tolerated.
Interpretation: Favipiravir does not accelerate viral clearance in early symptomatic COVID-19. The viral clearance rate estimated from quantitative measurements of oropharyngeal eluate viral densities assesses the antiviral efficacy of drugs in vivo with comparatively few studied patients.
Keywords: Antiviral efficacy; COVID-19; Early treatment; Favipiravir; Pharmacometrics; SARS-CoV-2.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Sissoko D, Laouenan C, Folkesson E, M'Lebing AB, Beavogui AH, Baize S, et al. Experimental treatment with favipiravir for ebola virus disease (the JIKI Trial): a historically controlled, single-arm proof-of-concept trial in Guinea. PLoS Med. 2016;13:e1001967. doi: 10.1371/journal.pmed.1001967. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

