Comparison of the protective effects of CS/TPP and CS/HPMCP nanoparticles containing berberine in ethanol-induced hepatotoxicity in rat
- PMID: 38225618
- PMCID: PMC10789080
- DOI: 10.1186/s12906-023-04318-9
Comparison of the protective effects of CS/TPP and CS/HPMCP nanoparticles containing berberine in ethanol-induced hepatotoxicity in rat
Abstract
Background: Alcoholic liver disease (ALD) is a globally critical condition with no available efficient treatments.
Methods: Herein, we generated chitosan (CS) nanoparticles cross-linked with two different agents, hydroxypropyl methylcellulose phthalate (HPMCP; termed as CS/HPMCP) and tripolyphosphate (TPP; termed as CS/TPP), and loaded them with berberine (BBr; referred to as CS/HPMCP/BBr and CS/TPP/BBr, respectively). Alongside the encapsulation efficiency (EE) and loading capacity (LC), the releasing activity of the nanoparticles was also measured in stimulated gastric fluid (SGF) and stimulated intestinal fluid (SIF) conditions. The effects of the prepared nanoparticles on the viability of mesenchymal stem cells (MSCs) were also evaluated. Ultimately, the protective effects of the nanoparticles were investigated in ALD mouse models.
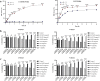
Results: SEM images demonstrated that CS/HPMCP and CS/TPP nanoparticles had an average size of 235.5 ± 42 and 172 ± 21 nm, respectively. The LC and EE for CS/HPMCP/BBr were calculated as 79.78% and 75.79%, respectively; while the LC and EE for CS/TPP/BBr were 84.26% and 80.05%, respectively. pH was a determining factor for releasing BBr from CS/HPMCP nanoparticles as a higher cargo-releasing rate was observed in a less acidic environment. Both the BBr-loaded nanoparticles increased the viability of MSCs in comparison with their BBr-free counterparts. In vivo results demonstrated CS/HPMCP/BBr and CS/TPP/BBr nanoparticles protected enzymatic liver functionality against ethanol-induced damage. They also prevented histopathological ethanol-induced damage.
Conclusions: Crosslinking CS nanoparticles with HPMCP can mediate controlled drug release in the intestine improving the bioavailability of BBr.
Keywords: Berberine; Chitosan; HPMCP; Hepatotoxicity; Nanoparticle; TPP.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Goldberg D, Ditah IC, Saeian K, Lalehzari M, Aronsohn A, Gorospe EC, et al. Changes in the prevalence of Hepatitis C virus Infection, nonalcoholic steatohepatitis, and alcoholic Liver Disease among patients with Cirrhosis or Liver Failure on the Waitlist for Liver Transplantation. Gastroenterology. 2017;152(5):1090–9e1. doi: 10.1053/j.gastro.2017.01.003. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

