Senolytic treatment alleviates doxorubicin-induced chemobrain
- PMID: 38225896
- PMCID: PMC10861213
- DOI: 10.1111/acel.14037
Senolytic treatment alleviates doxorubicin-induced chemobrain
Abstract
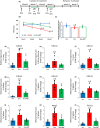
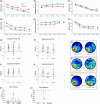
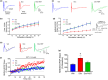
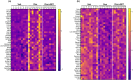
Doxorubicin (Dox), a widely used treatment for cancer, can result in chemotherapy-induced cognitive impairments (chemobrain). Chemobrain is associated with inflammation and oxidative stress similar to aging. As such, Dox treatment has also been used as a model of aging. However, it is unclear if Dox induces brain changes similar to that observed during aging since Dox does not readily enter the brain. Rather, the mechanism for chemobrain likely involves the induction of peripheral cellular senescence and the release of senescence-associated secretory phenotype (SASP) factors and these SASP factors can enter the brain to disrupt cognition. We examined the effect of Dox on peripheral and brain markers of aging and cognition. In addition, we employed the senolytic, ABT-263, which also has limited access to the brain. The results indicate that plasma SASP factors enter the brain, activating microglia, increasing oxidative stress, and altering gene transcription. In turn, the synaptic function required for memory was reduced in response to altered redox signaling. ABT-263 prevented or limited most of the Dox-induced effects. The results emphasize a link between cognitive decline and the release of SASP factors from peripheral senescent cells and indicate some differences as well as similarities between advanced age and Dox treatment.
Keywords: chemobrain; cognition; inflammation; oxidative stress; senolytic NMDA receptor; transcription.
© 2024 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
Daohong Zhou is an inventor of a patent for the discovery of ABT‐263 as a senolytic for the treatment of senescent cell‐associated diseases and a cofounder and a stockholder of Unity Biotechnology that develops senolytic therapy.
Figures
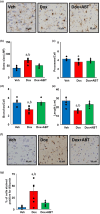
References
-
- Aird, K. M. , Iwasaki, O. , Kossenkov, A. V. , Tanizawa, H. , Fatkhutdinov, N. , Bitler, B. G. , Le, L. , Alicea, G. , Yang, T. L. , Johnson, F. B. , Noma, K. I. , & Zhang, R. G. (2016). HMGB2 orchestrates the chromatin landscape of senescence‐associated secretory phenotype gene loci. Journal of Cell Biology, 215(3), 325–334. 10.1083/jcb.201608026 - DOI - PMC - PubMed
-
- Ali, M. A. , Menze, E. T. , Tadros, M. G. , & Tolba, M. F. (2020). Caffeic acid phenethyl ester counteracts doxorubicin‐induced chemobrain in Sprague‐Dawley rats: Emphasis on the modulation of oxidative stress and neuroinflammation. Neuropharmacology, 181, 108334. 10.1016/j.neuropharm.2020.108334 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

