Kinetic Trapping of Photoluminescent Frameworks During High-Concentration Synthesis of Non-Emissive Metal-Organic Frameworks
- PMID: 38225948
- PMCID: PMC10788154
- DOI: 10.1021/acs.chemmater.3c02121
Kinetic Trapping of Photoluminescent Frameworks During High-Concentration Synthesis of Non-Emissive Metal-Organic Frameworks
Abstract
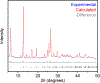
Metal-organic frameworks (MOFs) are porous, crystalline materials constructed from organic linkers and inorganic nodes with potential utility in gas separations, drug delivery, sensing, and catalysis. Small variations in MOF synthesis conditions can lead to a range of accessible frameworks with divergent chemical or photophysical properties. New methods to controllably access phases with tailored properties would broaden the scope of MOFs that can be reliably prepared for specific applications. Herein, we demonstrate that simply increasing the reaction concentration during the solvothermal synthesis of M2(dobdc) (M = Mg, Mn, Ni; dobdc4- = 2,5-dioxido-1,4-benzenedicarboxylate) MOFs unexpectedly leads to trapping of a new framework termed CORN-MOF-1 (CORN = Cornell University) instead. In-depth spectroscopic, crystallographic, and computational studies support that CORN-MOF-1 has a similar structure to M2(dobdc) but with partially protonated linkers and charge-balancing or coordinated formate groups in the pores. The resultant variation in linker spacings causes CORN-MOF-1 (Mg) to be strongly photoluminescent in the solid state, whereas H4dobdc and Mg2(dobdc) are weakly emissive due to excimer formation. In-depth photophysical studies suggest that CORN-MOF-1 (Mg) is the first MOF based on the H2dobdc2- linker that likely does not emit via an excited state intramolecular proton transfer (ESIPT) pathway. In addition, CORN-MOF-1 variants can be converted into high-quality samples of the thermodynamic M2(dobdc) phases by heating in N,N-dimethylformamide (DMF). Overall, our findings support that high-concentration synthesis provides a straightforward method to identify new MOFs with properties distinct from known materials and to produce highly porous samples of MOFs, paving the way for the discovery and gram-scale synthesis of framework materials.
Figures
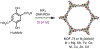






References
Grants and funding
LinkOut - more resources
Full Text Sources
