Plant molecules reinforce bone repair: Novel insights into phenol-modified bone tissue engineering scaffolds for the treatment of bone defects
- PMID: 38226013
- PMCID: PMC10788623
- DOI: 10.1016/j.mtbio.2023.100920
Plant molecules reinforce bone repair: Novel insights into phenol-modified bone tissue engineering scaffolds for the treatment of bone defects
Abstract
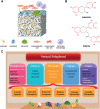
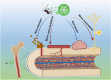
Bone defects have become a major cause of disability and death. To overcome the limitations of natural bone implants, including donor shortages and immune rejection risks, bone tissue engineering (BTE) scaffolds have emerged as a promising therapy for bone defects. Despite possessing good biocompatibility, these metal, ceramic and polymer-based scaffolds are still challenged by the harsh conditions in bone defect sites. ROS accumulation, bacterial infection, excessive inflammation, compromised blood supply deficiency and tumor recurrence negatively impact bone tissue cells (BTCs) and hinder the osteointegration of BTE scaffolds. Phenolic compounds, derived from plants and fruits, have gained growing application in treating inflammatory, infectious and aging-related diseases due to their antioxidant ability conferred by phenolic hydroxyl groups. The prevalent interactions between phenols and functional groups also facilitate their utilization in fabricating scaffolds. Consequently, phenols are increasingly incorporated into BTE scaffolds to boost therapeutic efficacy in bone defect. This review demonstrated the effects of phenols on BTCs and bone defect microenvironment, summarized the intrinsic mechanisms, presented the advances in phenol-modified BTE scaffolds and analyzed their potential risks in practical applications. Overall, phenol-modified BTE scaffolds hold great potential for repairing bone defects, offering novel patterns for BTE scaffold construction and advancing traumatological medicine.
Keywords: Bone defect; Bone tissue engineering; Osteogenesis; Phenols; Polyphenols; Scaffolds.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







Similar articles
-
Critical Overview on Pure Chitosan-based Scaffolds for Bone Tissue Engineering: Clinical insights in Dentistry.Int J Med Sci. 2023 Sep 18;20(12):1527-1534. doi: 10.7150/ijms.87978. eCollection 2023. Int J Med Sci. 2023. PMID: 37859701 Free PMC article. Review.
-
Chitosan-based 3D-printed scaffolds for bone tissue engineering.Int J Biol Macromol. 2021 Jul 31;183:1925-1938. doi: 10.1016/j.ijbiomac.2021.05.215. Epub 2021 Jun 6. Int J Biol Macromol. 2021. PMID: 34097956 Review.
-
GelMA-based bioactive hydrogel scaffolds with multiple bone defect repair functions: therapeutic strategies and recent advances.Biomater Res. 2023 Sep 15;27(1):86. doi: 10.1186/s40824-023-00422-6. Biomater Res. 2023. PMID: 37715230 Free PMC article. Review.
-
Stem Cell-Seeded 3D-Printed Scaffolds Combined with Self-Assembling Peptides for Bone Defect Repair.Tissue Eng Part A. 2022 Feb;28(3-4):111-124. doi: 10.1089/ten.TEA.2021.0055. Epub 2021 Dec 30. Tissue Eng Part A. 2022. PMID: 34157886
-
Recent advances in carbon dots: synthesis and applications in bone tissue engineering.Nanoscale. 2023 Feb 16;15(7):3106-3119. doi: 10.1039/d2nr05951g. Nanoscale. 2023. PMID: 36723029 Review.
Cited by
-
Microenvironment-responsive nanoparticles functionalized titanium implants mediate redox balance and immunomodulation for enhanced osseointegration.Mater Today Bio. 2025 Mar 2;31:101628. doi: 10.1016/j.mtbio.2025.101628. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 40124346 Free PMC article.
-
Development of polyvinyl alcohol nanofiber scaffolds loaded with flaxseed extract for bone regeneration: phytochemicals, cell proliferation, adhesion, and osteogenic gene expression.Front Chem. 2024 Jul 31;12:1417407. doi: 10.3389/fchem.2024.1417407. eCollection 2024. Front Chem. 2024. PMID: 39144698 Free PMC article.
-
A 3D-printed scaffold composed of Alg/HA/SIS for the treatment of diabetic bone defects.J Orthop Translat. 2024 Jul 25;48:25-38. doi: 10.1016/j.jot.2024.07.006. eCollection 2024 Sep. J Orthop Translat. 2024. PMID: 39087140 Free PMC article.
-
Antioxidant scaffolds for enhanced bone regeneration: recent advances and challenges.Biomed Eng Online. 2025 Apr 8;24(1):41. doi: 10.1186/s12938-025-01370-z. Biomed Eng Online. 2025. PMID: 40200302 Free PMC article. Review.
References
-
- Allesina L., Mazzola M., Belluati A., Mosca S., Placella G., Salini V. Surgical treatment of critical size bone defects with Masquelet technique versus bone transport: a systematic review and meta-analysis of comparative studies. Arch. Orthop. Trauma Surg. 2023;143:7081–7096. doi: 10.1007/s00402-023-05049-9. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

