Clinical efficacy and satisfaction of a digital wheeze detector in a multicentre randomised controlled trial: the WheezeScan study
- PMID: 38226060
- PMCID: PMC10789262
- DOI: 10.1183/23120541.00518-2023
Clinical efficacy and satisfaction of a digital wheeze detector in a multicentre randomised controlled trial: the WheezeScan study
Abstract
Introduction: Wheezing is common in preschool children and its clinical assessment often challenging for caretakers. This study aims to evaluate the impact of a novel digital wheeze detector (WheezeScan™) on disease control in a home care setting.
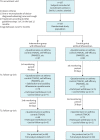
Methods: A multicentre randomised open-label controlled trial was conducted in Berlin, Istanbul and London. Participants aged 4-84 months with a doctor's diagnosis of recurrent wheezing in the past 12 months were included. While the control group followed usual care, the intervention group received the WheezeScan™ for at-home use for 120 days. Parents completed questionnaires regarding their child's respiratory symptoms, disease-related and parental quality of life, and caretaker self-efficacy at baseline (T0), 90 days (T1) and 4 months (T2).
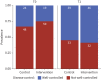
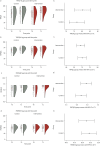
Results: A total of 167 children, with a mean±sd age of 3.2±1.6 years, were enrolled in the study (intervention group n=87; control group n=80). There was no statistically significant difference in wheeze control assessed by TRACK (mean difference 3.8, 95% CI -2.3-9.9; p=0.2) at T1 between treatment groups (primary outcome). Children's and parental quality of life and parental self-efficacy were comparable between both groups at T1. The evaluation of device usability and perception showed that parents found it useful.
Conclusion: In the current study population, the wheeze detector did not show significant impact on the home management of preschool wheezing. Hence, further research is needed to better understand how the perception and usage behaviour may influence the clinical impact of a digital support.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: T-M. Weichert, S. Roßberg, C. Grenzbach, E. Dellbrügger, B. Karadag and A. Whitehouse have received support for the work on this study via an unrestricted scientific grant. U. Grittner has received support for her work on this study as a biostatistician from OMRON Healthcare Co., Ltd via an unrestricted scientific grant. S. Dramburg and J. Grigg received personal fees from OMRON Healthcare. P.M. Matricardi has received funding and provision of study material from OMRON Health Care for the present study as well as consulting fees from OMRON Healthcare not related to the present study. C.J. Hernandez Toro, Y.H. Do, A. Pizulli, W. van Aalderen and E. Haarman have nothing to disclose.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous
