Real-world experience of nintedanib for progressive fibrosing interstitial lung disease in the UK
- PMID: 38226064
- PMCID: PMC10789269
- DOI: 10.1183/23120541.00529-2023
Real-world experience of nintedanib for progressive fibrosing interstitial lung disease in the UK
Abstract
Background: Nintedanib slows progression of lung function decline in patients with progressive fibrosing (PF) interstitial lung disease (ILD) and was recommended for this indication within the United Kingdom (UK) National Health Service in Scotland in June 2021 and in England, Wales and Northern Ireland in November 2021. To date, there has been no national evaluation of the use of nintedanib for PF-ILD in a real-world setting.
Methods: 26 UK centres were invited to take part in a national service evaluation between 17 November 2021 and 30 September 2022. Summary data regarding underlying diagnosis, pulmonary function tests, diagnostic criteria, radiological appearance, concurrent immunosuppressive therapy and drug tolerability were collected via electronic survey.
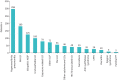
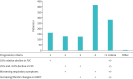
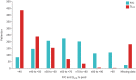
Results: 24 UK prescribing centres responded to the service evaluation invitation. Between 17 November 2021 and 30 September 2022, 1120 patients received a multidisciplinary team recommendation to commence nintedanib for PF-ILD. The most common underlying diagnoses were hypersensitivity pneumonitis (298 out of 1120, 26.6%), connective tissue disease associated ILD (197 out of 1120, 17.6%), rheumatoid arthritis associated ILD (180 out of 1120, 16.0%), idiopathic nonspecific interstitial pneumonia (125 out of 1120, 11.1%) and unclassifiable ILD (100 out of 1120, 8.9%). Of these, 54.4% (609 out of 1120) were receiving concomitant corticosteroids, 355 (31.7%) out of 1120 were receiving concomitant mycophenolate mofetil and 340 (30.3%) out of 1120 were receiving another immunosuppressive/modulatory therapy. Radiological progression of ILD combined with worsening respiratory symptoms was the most common reason for the diagnosis of PF-ILD.
Conclusion: We have demonstrated the use of nintedanib for the treatment of PF-ILD across a broad range of underlying conditions. Nintedanib is frequently co-prescribed alongside immunosuppressive and immunomodulatory therapy. The use of nintedanib for the treatment of PF-ILD has demonstrated acceptable tolerability in a real-world setting.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: A.J. Simpson has received funding to his institution from Boehringer Ingelheim (BI) to undertake an educational meeting. A. West has received support from BI for speaking at or chairing educational events, and attendance and travel to educational meetings; and is part of an advisory board for BI and Avalyn Pharmaceuticals. A. John has received funding from BI to attend an educational event. A.M. Wilson has received grants from Aseptika, Brainomix and BASF, has received speakers’ fees from BI, has received support for attending meetings by Chiesi, and has institutional interests with Celgene Corporation, GSK and Insmed Inc. A. Crawshaw has received speakers’ fees from BI and AstraZeneca (AZ). A.U. Wells has undertaken advisory board activity and consultant work for BI, Roche and Veracyte. C.C. Huntley has received an honorarium for educational content from BI and sponsorship for conference attendance. D. Dosanjh has received a speaker's fee from BI, meeting attendance costs from AZ and is part of the advisory board for AZ, Gilead, BI and Synairgen. E. Renzoni has received institutional funding, honoraria for educational events and funding for conference attendance from BI, and is member of the advisory board for BI and Roche. F. Chua has received consulting fees, honoraria, support for conference attendance and is an advisory board member for BI. G. Saini has received institutional payment for educational presentation from BI. G. Dixon, H. Stone, L.M. Nicol and I.A. Forrest have received support for educational event attendance from BI. J.C.L. Rodrigues has received grant funding from NIHR, consulting fees from NHSx and HeartFlow, honoraria from Sanofi, Aidence and 4-C Research market research, meeting attendance support from Aidence and HeartFlow, leadership role in Heart and Lung Imaging LTD (HLH), stock in Radnet and shares in HLH. K. Tsaneva-Atanasova has financial support from EPSRC grant. M. Naqvi has received a grant from NHS Digital, honoraria from BI, AZ and Roche, support for meeting attendance from BI and advisory board membership for BI, and is ILD Pharmacist Network Chair and ILD-IN Co-chair. M.G. Jones has received grants from Royal Society, BI, NC3Rs, MRC, AAIR Charity and the British Lung Foundation. P.M. George has received an institutional grant from BI, honoraria from BI, Roche, Teva, Cipla and Brainomix, meeting attendance support from BI and Roche and has stock in Brainomix. P. Molyneaux has grant funding from AZ, consulting fees from Roche, BI, AZ, Trevi and Qureight, and honoraria from BI and Roche; and is an associate editor of this journal. P. Rivera-Ortega has received grant funding from MRC, institutional grant funding from BI, Roche, CSL Behring, Fibrogen, Vicore Pharma AB, Gilead Sciences and Galecto, consulting fees from BI and Roche, honoraria from BI, Roche and Respiratory Effectiveness Group (REG), support for meeting attendance from BI and REG, is a chair of the REG and member of the Global Writing Group Committee for REMAP-ILD. R.K. Coker has received honoraria from BI. S. Agnew has received honoraria from BI, support for meeting attendance from BI and is member of the BTS ILD registry advisory board. S.L. Barratt has received consulting fees and honoraria from BI. S. Hart has received research grant from BI, consulting fees from Trevi Therapeutics, honoraria and support for meeting attendance from BI and Chiesi, was Chair of the BTS Standard of Care Committee 2019–2022, and is a Trustee of Action for Pulmonary Fibrosis and an associate editor of this journal. S. Barth received honoraria from BI for educational meeting facilitating. T. Garfoot received support to attend the ILD IN annual conference. T. Gatheral has received speakers’ fees from BI. Conflict of interest: The remaining authors have no competing interests.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
