Therapeutic TNF Inhibitors Exhibit Differential Levels of Efficacy in Accelerating Cutaneous Wound Healing
- PMID: 38226320
- PMCID: PMC10788510
- DOI: 10.1016/j.xjidi.2023.100250
Therapeutic TNF Inhibitors Exhibit Differential Levels of Efficacy in Accelerating Cutaneous Wound Healing
Abstract
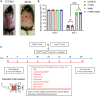
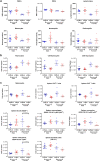
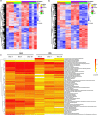
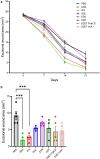
Adalimumab but neither etanercept nor certolizumab-pegol has been reported to induce a wound-healing profile in vitro by regulating macrophage differentiation and matrix metalloproteinase expression, which may underlie the differences in efficacy between various TNF-α inhibitors in impaired wound healing in patients with hidradenitis suppurativa, a chronic inflammatory skin disease. To examine and compare the efficacy of various TNF inhibitors in cutaneous wound healing in vivo, a human TNF knock-in Leprdb/db mouse model was established to model the impaired cutaneous wound healing as seen in hidradenitis suppurativa. The vehicle group exhibited severe impairments in cutaneous wound healing. In contrast, adalimumab significantly accelerated healing, confirmed by both histologic assessment and a unique healing transcriptional profile. Moreover, adalimumab and infliximab showed similar levels of efficacy, but golimumab was less effective, along with etanercept and certolizumab-pegol. In line with histologic assessments, proteomics analyses from healing wounds exposed to various TNF inhibitors revealed distinct and differential wound-healing signatures that may underlie the differential efficacy of these inhibitors in accelerating cutaneous wound healing. Taken together, these data revealed that TNF inhibitors exhibited differential levels of efficacy in accelerating cutaneous wound healing in the impaired wound-healing model in vivo.
Keywords: Hidradenitis suppurativa; TNF inhibitors; Wound healing.
© 2023 The Authors.
Figures



References
-
- Adams D.R., Yankura J.A., Fogelberg A.C., Anderson B.E. Treatment of hidradenitis suppurativa with Etanercept injection. Arch Dermatol. 2010;146:501–504. - PubMed
-
- Butler D.M., Malfait A.M., Mason L.J., Warden P.J., Kollias G., Maini R.N., et al. DBA/1 mice expressing the human TNF-alpha transgene develop a severe, erosive arthritis: characterization of the cytokine cascade and cellular composition. J Immunol. 1997;159:2867–2876. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

