Cytidine Acetylation Across the Tree of Life
- PMID: 38226431
- PMCID: PMC11578069
- DOI: 10.1021/acs.accounts.3c00673
Cytidine Acetylation Across the Tree of Life
Abstract
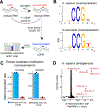
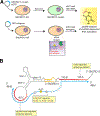
Acetylation plays a critical role in regulating eukaryotic transcription via the modification of histones. Beyond this well-documented function, a less explored biological frontier is the potential for acetylation to modify and regulate the function of RNA molecules themselves. N4-Acetylcytdine (ac4C) is a minor RNA nucleobase conserved across all three domains of life (archaea, bacteria, and eukarya), a conservation that suggests a fundamental role in biological processes. Unlike many RNA modifications that are controlled by large enzyme families, almost all organisms catalyze ac4C using a homologue of human Nat10, an essential disease-associated acetyltransferase enzyme.A critical step in defining the fundamental functions of RNA modifications has been the development of methods for their sensitive and specific detection. This Account describes recent progress enabling the use of chemical sequencing reactions to map and quantify ac4C with single-nucleotide resolution in RNA. To orient readers, we first provide historical background of the discovery of ac4C and the enzymes that catalyze its formation. Next, we describe mechanistic experiments that led to the development of first- and second-generation sequencing reactions able to determine ac4C's position in a polynucleotide by exploiting the nucleobase's selective susceptibility to reduction by hydride donors. A notable feature of this chemistry, which may serve as a prototype for nucleotide resolution RNA modification sequencing reactions more broadly, is its ability to drive a penetrant and detectable gain of signal specifically at ac4C sites. Emphasizing practical applications, we present how this optimized chemistry can be integrated into experimental workflows capable of sensitive, transcriptome-wide analysis. Such readouts can be applied to quantitatively define the ac4C landscape across the tree of life. For example, in human cell lines and yeast, this method has uncovered that ac4C is highly selective, predominantly occupying dominant sites within rRNA (rRNA) and tRNA (tRNA). By contrast, when we extend these analyses to thermophilic archaea they identify the potential for much more prevalent patterns of cytidine acetylation, leading to the discovery of a role for this modification in adaptation to environmental stress. Nucleotide resolution analyses of ac4C have also allowed for the determination of structure-activity relationships required for short nucleolar RNA (snoRNA)-catalyzed ac4C deposition and the discovery of organisms with unexpectedly divergent tRNA and rRNA acetylation signatures. Finally, we share how these studies have shaped our approach to evaluating novel ac4C sites reported in the literature and highlight unanswered questions and new directions that set the stage for future research in the field.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




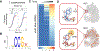
References
-
- Sas-Chen A; Thomas JM; Matzov D; Taoka M; Nance KD; Nir R; Bryson KM; Shachar R; Liman GLS; Burkhart BW; Thalalla Gamage S; Nobe Y; Briney CA; Levy MJ; Fuchs RT; Robb GB; Hartmann J; Sharma S; Lin Q; Florens L; Washburn MP; Isobe T; Santangelo TJ; Shalev-Benami M; Meier JL; Schwartz S Dynamic RNA acetylation revealed by quantitative cross-evolutionary mapping. Nature 2020, 583, 638–643. - PMC - PubMed
-
- Bortolin-Cavaille ML; Quillien A; Thalalla Gamage S; Thomas JM; Sas-Chen A; Sharma S; Plisson-Chastang C; Vandel L; Blader P; Lafontaine DLJ; Schwartz S; Meier JL; Cavaille J Probing small ribosomal subunit RNA helix 45 acetylation across eukaryotic evolution. Nucleic Acids Res. 2022, 50, 6284–6299. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

