Intestinal P-gp activity is reduced in postmenopausal women under breast cancer therapy
- PMID: 38226443
- PMCID: PMC10801393
- DOI: 10.1111/cts.13713
Intestinal P-gp activity is reduced in postmenopausal women under breast cancer therapy
Abstract
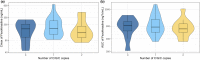
Intestinal P-glycoprotein (P-gp) activity plays a crucial role in modulating the oral bioavailability of its substrates. Fexofenadine has commonly been used as a P-gp probe, although it is important to note the involvement of other drug transporters like, OATP1B1, OATP1B3, and OATP2B1. In vitro studies demonstrated an upregulation of P-gp protein in response to exposure to pregnancy-related hormones. The objective of this study was to investigate how intestinal P-gp activity is impacted by menopausal status. This study sampled fexofenadine plasma concentrations over 0-12 h after probe drug administration from two groups of patients with breast cancer: premenopausal (n = 20) and postmenopausal (n = 20). Fexofenadine plasma concentrations were quantified using liquid-chromatography tandem mass spectrometry. Area under the plasma concentration-time curve from zero to infinity (AUCinf ) was calculated through limited sampling strategies equation. Multiple linear regression was applied on AUCinf , maximum plasma concentration (Cmax ), and time to Cmax . Postmenopausal patients showed a significant increase in Cmax (geometric mean and 95% confidence interval [CI] 143.54, 110.95-176.13 vs. 223.54 ng/mL, 161.02-286.06 and in AUCinf 685.55, 534.98-878.50 vs. 933.54 ng·h/mL 735.45-1184.99) compared to premenopausal patients. The carriers of the ABCB1 3435 allele T displayed higher Cmax values of 166.59 (95% CI: 129.44-214.39) compared to the wild type at 147.47 ng/mL (95% CI: 111.91-194.34, p = 0.02). In postmenopausal individuals, the decrease in P-gp activity of ~40% may lead to an increased plasma exposure of orally administered P-gp substrates.
© 2024 The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures


References
-
- Madla CM, Gavins FKH, Merchant HA, Orlu M, Murdan S, Basit AW. Let's talk about sex: differences in drug therapy in males and females. Adv Drug Deliv Rev. 2021;175:113804. - PubMed
-
- Paine MF, Ludington SS, Chen ML, Stewart PW, Huang SM, Watkins PB. Do men and women differ in proximal small intestinal CYP3A or P‐glycoprotein expression? Drug Metab Dispos. 2005;33(3):426‐433. - PubMed
-
- Harris RZ, Tsunoda SM, Mroczkowski P, Wong H, Benet LZ. The effects of menopause and hormone replacement therapies on prednisolone and erythromycin pharmacokinetics. Clin Pharmacol Ther. 1996;59(4):429‐435. - PubMed
-
- Nazir S, Iqbal Z, Nasir F. Impact of menopause on pharmacokinetics of rosuvastatin compared with premenopausal women. Eur J Drug Metab Pharmacokinet. 2016;41(5):505‐509. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

