CAR T cells for hematological malignancies
- PMID: 38226627
- PMCID: PMC10786683
- DOI: 10.1172/JCI177160
CAR T cells for hematological malignancies
Conflict of interest statement
Figures
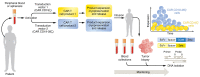
References
-
- Eshhar Z, et al. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90(2):720–724. doi: 10.1073/pnas.90.2.720. - DOI - PMC - PubMed

