Imaging chronic active lesions in multiple sclerosis: a consensus statement
- PMID: 38226694
- PMCID: PMC11370808
- DOI: 10.1093/brain/awae013
Imaging chronic active lesions in multiple sclerosis: a consensus statement
Abstract
Chronic active lesions (CAL) are an important manifestation of chronic inflammation in multiple sclerosis and have implications for non-relapsing biological progression. In recent years, the discovery of innovative MRI and PET-derived biomarkers has made it possible to detect CAL, and to some extent quantify them, in the brain of persons with multiple sclerosis, in vivo. Paramagnetic rim lesions on susceptibility-sensitive MRI sequences, MRI-defined slowly expanding lesions on T1-weighted and T2-weighted scans, and 18-kDa translocator protein-positive lesions on PET are promising candidate biomarkers of CAL. While partially overlapping, these biomarkers do not have equivalent sensitivity and specificity to histopathological CAL. Standardization in the use of available imaging measures for CAL identification, quantification and monitoring is lacking. To fast-forward clinical translation of CAL, the North American Imaging in Multiple Sclerosis Cooperative developed a consensus statement, which provides guidance for the radiological definition and measurement of CAL. The proposed manuscript presents this consensus statement, summarizes the multistep process leading to it, and identifies the remaining major gaps in knowledge.
Keywords: MRI-defined slowly evolving lesions; chronic active lesions; iron; microglia; multiple sclerosis; paramagnetic rim lesions.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com. Elements of this work were written by employees of the US Government.
Conflict of interest statement
M.A. received consultancy income from Sanofi, Biogen, Abata Therapeutics and Glaxo Smith Klein. C.J.A. has received consulting income from Horizon Therapeutics, Genentech, Sanofi Genzyme, Alexion, EMD Serono, and Novartis; compensation for scientific reviewing from the Department of Defense; and honoraria related to creating educational content for Projects in Knowledge, Catamount Education, and Spire Learning. F.B. received consultancy income from Sanofi-Genzyme; Biogen, Janssen Pharmaceuticals and EMD Serono and receives compensation for scientific reviewing from the National Institutes of Health. P.A.C. received consulting honoraria for serving on SABs for Nervgen, Idorsia, Biogen, Vaccitech, and Lilly. C.E. is an employee of NeuroRx Research and received speaking honoraria from EMD Serono
Figures




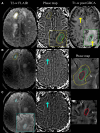
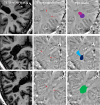
References
-
- Katz D, Taubenberger JK, Cannella B, McFarlin DE, Raine CS, McFarland HF. Correlation between magnetic resonance imaging findings and lesion development in chronic, active multiple sclerosis. Ann Neurol. 1993;34:661–669. - PubMed
-
- McFarland HF. The lesion in multiple sclerosis: Clinical, pathological, and magnetic resonance imaging considerations. J Neurol Neurosurg Psychiatry. 1998;64(S1):S26–S30. - PubMed
-
- Harris JO, Frank JA, Patronas N, McFarlin DE, McFarland HF. Serial gadolinium-enhanced magnetic resonance imaging scans in patients with early, relapsing-remitting multiple sclerosis: Implications for clinical trials and natural history. Ann Neurol. 1991;29:548–555. - PubMed
-
- Stone LA, Smith ME, Albert PS, et al. Blood-brain barrier disruption on contrast-enhanced MRI in patients with mild relapsing-remitting multiple sclerosis: Relationship to course, gender, and age. Neurology. 1995;45:1122–1126. - PubMed
-
- Smith ME, Stone LA, Albert PS, et al. Clinical worsening in multiple sclerosis is associated with increased frequency and area of gadopentetate dimeglumine-enhancing magnetic resonance imaging lesions. Ann Neurol. 1993;33:480–489. - PubMed
Publication types
MeSH terms
Grants and funding
- Roche-Genentech
- MS-1610-37047/Patient Centered Outcomes Research Institute
- RG-1802-30140/National MS Society
- 1750327/Fondazione Regionale per la Ricerca Biomedica Early Career Award
- R01 NS118886/NS/NINDS NIH HHS/United States
- RG-1707-28775/Atara
- R01 EB007258/EB/NIBIB NIH HHS/United States
- R01 NS112907/NS/NINDS NIH HHS/United States
- R21 NS129197/NS/NINDS NIH HHS/United States
- KL2TR001454/TR/NCATS NIH HHS/United States
- 1R01NS122980-01/NIH-NINDS
- R01 NS040801/NS/NINDS NIH HHS/United States
- R21 NS123419/NS/NINDS NIH HHS/United States
- MS Society of Canada
- R01 NS112274/NS/NINDS NIH HHS/United States
- R01 MH112847/MH/NIMH NIH HHS/United States
- R01 MH123550/MH/NIMH NIH HHS/United States
- U01NS116776-01/NH/NIH HHS/United States
- R01 NS122980/NS/NINDS NIH HHS/United States
- I01CX002160-01A1/Veterans Health Administration
- I01 CX002160/CX/CSRD VA/United States
- R01 DA047088/DA/NIDA NIH HHS/United States
- 1U01NS116776-01/Brain Canada
- TL1 TR001454/TR/NCATS NIH HHS/United States
- K23 NS126718/NS/NINDS NIH HHS/United States
- R01 NS102267/NS/NINDS NIH HHS/United States
- R01 NS104403/NS/NINDS NIH HHS/United States
- R21 NS116434/NS/NINDS NIH HHS/United States
- 17313/Conrad N. Hilton Foundation
- R01 NS082347/NS/NINDS NIH HHS/United States
- 2019-1677/Cariplo Foundation
- R35 NS097303/NS/NINDS NIH HHS/United States
- Natural Sciences and Engineering Research Council of Canada
- R56 NR019306/NR/NINR NIH HHS/United States
- R01 NS 112274/Erwin Rautenberg Foundation
- 1R21NS1226737-01/International Collaboration on Repair Discoveries
- MS210103/Department of Defense
- PA-2107-38081/International Progressive MS Alliance
- R01 NS104283/NS/NINDS NIH HHS/United States
- Z01 NS003119/Intramural Research Program of NIH-NINDS
- RFA-2203-39325/NMSS Society
- R01 NS060910/NS/NINDS NIH HHS/United States
- Craig H. Neilsen Foundation
- U01 NS116776/NS/NINDS NIH HHS/United States
- R01NS082347/Voros Innovation Impact Funds
- Principia
LinkOut - more resources
Full Text Sources
Medical

