Antiviral action of a functionalized plastic surface against human coronaviruses
- PMID: 38226803
- PMCID: PMC10846231
- DOI: 10.1128/spectrum.03008-23
Antiviral action of a functionalized plastic surface against human coronaviruses
Abstract
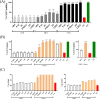
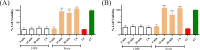
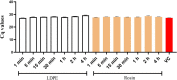
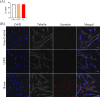
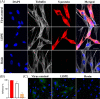
Viruses may persist on solid surfaces for long periods, which may contribute to indirect transmission. Thus, it is imperative to develop functionalized surfaces that will lower the infectious viral load in everyday life. Here, we have tested a plastic surface functionalized with tall oil rosin against the seasonal human coronavirus OC43 as well as severe acute respiratory syndrome coronavirus 2. All tested non-functionalized plastic surfaces showed virus persistence up to 48 h. In contrast, the functionalized plastic showed good antiviral action already within 15 min of contact and excellent efficacy after 30 min over 90% humidity. Excellent antiviral effects were also observed at lower humidities of 20% and 40%. Despite the hydrophilic nature of the functionalized plastic, viruses did not adhere strongly to it. According to helium ion microscopy, viruses appeared flatter on the rosin-functionalized surface, but after flushing away from the rosin-functionalized surface, they showed no apparent structural changes when imaged by transmission electron microscopy of cryogenic or negatively stained specimens or by atomic force microscopy. Flushed viruses were able to bind to their host cell surface and enter endosomes, suggesting that the fusion with the endosomal membrane was halted. The eluted rosin from the functionalized surface demonstrated its ability to inactivate viruses, indicating that the antiviral efficacy relied on the active leaching of the antiviral substances, which acted on the viruses coming into contact. The rosin-functionalized plastic thus serves as a promising candidate as an antiviral surface for enveloped viruses.IMPORTANCEDuring seasonal and viral outbreaks, the implementation of antiviral plastics can serve as a proactive strategy to limit the spread of viruses from contaminated surfaces, complementing existing hygiene practices. In this study, we show the efficacy of a rosin-functionalized plastic surface that kills the viral infectivity of human coronaviruses within 15 min of contact time, irrespective of the humidity levels. In contrast, non-functionalized plastic surfaces retain viral infectivity for an extended period of up to 48 h. The transient attachment on the surface or the leached active components do not cause major structural changes in the virus or prevent receptor binding; instead, they effectively block viral infection at the endosomal stage.
Keywords: antiviral surface; human coronavirus; plastic; tall oil rosin; virus persistence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

