Phase I Study of ORIC-101, a Glucocorticoid Receptor Antagonist, in Combination with Enzalutamide in Patients with Metastatic Castration-resistant Prostate Cancer Progressing on Enzalutamide
- PMID: 38226958
- PMCID: PMC10947849
- DOI: 10.1158/1078-0432.CCR-23-3508
Phase I Study of ORIC-101, a Glucocorticoid Receptor Antagonist, in Combination with Enzalutamide in Patients with Metastatic Castration-resistant Prostate Cancer Progressing on Enzalutamide
Abstract
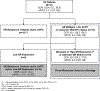
Purpose: Increased glucocorticoid receptor (GR) signaling is a proposed compensatory mechanism of resistance to androgen receptor (AR) inhibition in metastatic castration-resistant prostate cancer (mCRPC). ORIC-101 is a potent and selective orally-bioavailable GR antagonist.
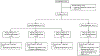
Patients and methods: Safety, pharmacokinetic/pharmacodynamic, and antitumor activity of ORIC-101 in combination with enzalutamide were studied in patients with mCRPC progressing on enzalutamide. ORIC-101 doses ranging from 80 to 240 mg once daily were tested in combination with enzalutamide 160 mg once daily. Pharmacokinetics/pharmacodynamics was assessed after a single dose and at steady state. Disease control rate (DCR) at 12 weeks was evaluated at the recommended phase 2 dose (RP2D).
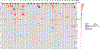
Results: A total of 41 patients were enrolled. There were no dose-limiting toxicities and the RP2D was selected as 240 mg of ORIC-101 and 160 mg of enzalutamide daily. At the RP2D, the most common treatment-related adverse events were fatigue (38.7%), nausea (29.0%), decreased appetite (19.4%), and constipation (12.9%). Pharmacokinetic/pharmacodynamic data confirmed ORIC-101 achieved exposures necessary for GR target engagement. Overall, for 31 patients treated at the RP2D, there was insufficient clinical benefit based on DCR (25.8%; 80% confidence interval: 15.65-38.52) which did not meet the prespecified target rate, leading to termination of the study. Exploratory subgroup analyses based on baseline GR expression, presence of AR resistance variants, and molecular features of aggressive variant prostate cancer suggested possible benefit in patients with high GR expression and no other resistance markers, although this would require confirmation.
Conclusions: Although the combination of ORIC-101 and enzalutamide demonstrated an acceptable tolerability profile, GR target inhibition with ORIC-101 did not produce clinical benefit in men with metastatic prostate cancer resistant to enzalutamide.
Trial registration: ClinicalTrials.gov NCT04033328.
©2024 American Association for Cancer Research.
Conflict of interest statement
Conflicts of Interest:
Figures
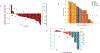

Similar articles
-
Talazoparib plus enzalutamide in men with metastatic castration-resistant prostate cancer: final overall survival results from the randomised, placebo-controlled, phase 3 TALAPRO-2 trial.Lancet. 2025 Aug 2;406(10502):447-460. doi: 10.1016/S0140-6736(25)00684-1. Epub 2025 Jul 16. Lancet. 2025. PMID: 40683290 Clinical Trial.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
rechARge: a randomized phase III trial of the androgen receptor ligand-directed degrader, BMS-986365, vs investigator's choice in patients with mCRPC.Future Oncol. 2025 Jun;21(14):1771-1777. doi: 10.1080/14796694.2025.2502318. Epub 2025 Jun 2. Future Oncol. 2025. PMID: 40455815 Free PMC article.
-
Cost-effectiveness of enzalutamide with androgen-deprivation therapy (ADT) versus ADT alone for the treatment of high-risk biochemically recurrent non-metastatic castration-sensitive prostate cancer in Canada.J Med Econ. 2025 Dec;28(1):766-777. doi: 10.1080/13696998.2025.2503660. Epub 2025 May 23. J Med Econ. 2025. PMID: 40395149
-
Comparative efficacy of second-generation androgen receptor inhibitors for treating prostate cancer: A systematic review and network meta-analysis.Front Endocrinol (Lausanne). 2023 Mar 9;14:1134719. doi: 10.3389/fendo.2023.1134719. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36967752 Free PMC article.
Cited by
-
A Phase I Trial of Enzalutamide Plus Selective Glucocorticoid Receptor Modulator Relacorilant in Patients with Metastatic Castration-Resistant Prostate Cancer.Clin Cancer Res. 2024 Jun 3;30(11):2384-2392. doi: 10.1158/1078-0432.CCR-23-3636. Clin Cancer Res. 2024. PMID: 38536082 Free PMC article. Clinical Trial.
-
ORIC-101, a Glucocorticoid Receptor Antagonist, in Combination with Nab-Paclitaxel in Patients with Advanced Solid Tumors.Cancer Res Commun. 2024 Sep 1;4(9):2415-2426. doi: 10.1158/2767-9764.CRC-24-0115. Cancer Res Commun. 2024. PMID: 39177285 Free PMC article. Clinical Trial.
-
Emerging frontiers in androgen receptor research for prostate Cancer: insights from the 2nd international androgen receptor Symposium.J Exp Clin Cancer Res. 2024 Jul 17;43(1):194. doi: 10.1186/s13046-024-03125-5. J Exp Clin Cancer Res. 2024. PMID: 39014480 Free PMC article.
-
Glucocorticoid receptor action in prostate cancer: the role of transcription factor crosstalk.Front Endocrinol (Lausanne). 2024 Jul 4;15:1437179. doi: 10.3389/fendo.2024.1437179. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39027480 Free PMC article. Review.
-
Enzalutamide inhibits PEX10 function and sensitizes prostate cancer cells to ROS activators.Cell Death Dis. 2024 Aug 3;15(8):559. doi: 10.1038/s41419-024-06937-7. Cell Death Dis. 2024. PMID: 39097593 Free PMC article.
References
-
- Azher S, Azami O, Amato C, McCullough M, Celentano A, Cirillo N. The non-conventional effects of glucocorticoids in cancer. J Cell Physiol. 2016; 231:2368–2373 - PubMed
-
- Puhr M, Hoefer J, Eigentler A, Ploner C, Handle F, Schaefer G, et al. The glucocorticoid receptor is a key player for prostate cancer cell survival and a target for improved antiandrogen therapy. Clin Cancer Res. 2018;24:927–938. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

