Analysis of Breast Cancer Mortality in the US-1975 to 2019
- PMID: 38227031
- PMCID: PMC10792466
- DOI: 10.1001/jama.2023.25881
Analysis of Breast Cancer Mortality in the US-1975 to 2019
Abstract
Importance: Breast cancer mortality in the US declined between 1975 and 2019. The association of changes in metastatic breast cancer treatment with improved breast cancer mortality is unclear.
Objective: To simulate the relative associations of breast cancer screening, treatment of stage I to III breast cancer, and treatment of metastatic breast cancer with improved breast cancer mortality.
Design, setting, and participants: Using aggregated observational and clinical trial data on the dissemination and effects of screening and treatment, 4 Cancer Intervention and Surveillance Modeling Network (CISNET) models simulated US breast cancer mortality rates. Death due to breast cancer, overall and by estrogen receptor and ERBB2 (formerly HER2) status, among women aged 30 to 79 years in the US from 1975 to 2019 was simulated.
Exposures: Screening mammography, treatment of stage I to III breast cancer, and treatment of metastatic breast cancer.
Main outcomes and measures: Model-estimated age-adjusted breast cancer mortality rate associated with screening, stage I to III treatment, and metastatic treatment relative to the absence of these exposures was assessed, as was model-estimated median survival after breast cancer metastatic recurrence.
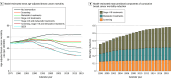
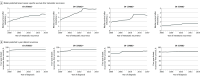
Results: The breast cancer mortality rate in the US (age adjusted) was 48/100 000 women in 1975 and 27/100 000 women in 2019. In 2019, the combination of screening, stage I to III treatment, and metastatic treatment was associated with a 58% reduction (model range, 55%-61%) in breast cancer mortality. Of this reduction, 29% (model range, 19%-33%) was associated with treatment of metastatic breast cancer, 47% (model range, 35%-60%) with treatment of stage I to III breast cancer, and 25% (model range, 21%-33%) with mammography screening. Based on simulations, the greatest change in survival after metastatic recurrence occurred between 2000 and 2019, from 1.9 years (model range, 1.0-2.7 years) to 3.2 years (model range, 2.0-4.9 years). Median survival for estrogen receptor (ER)-positive/ERBB2-positive breast cancer improved by 2.5 years (model range, 2.0-3.4 years), whereas median survival for ER-/ERBB2- breast cancer improved by 0.5 years (model range, 0.3-0.8 years).
Conclusions and relevance: According to 4 simulation models, breast cancer screening and treatment in 2019 were associated with a 58% reduction in US breast cancer mortality compared with interventions in 1975. Simulations suggested that treatment for stage I to III breast cancer was associated with approximately 47% of the mortality reduction, whereas treatment for metastatic breast cancer was associated with 29% of the reduction and screening with 25% of the reduction.
Conflict of interest statement
Figures

Comment in
-
Benefits of Breast Cancer Screening and Treatment on Mortality.JAMA. 2024 Jan 16;331(3):199-200. doi: 10.1001/jama.2023.26730. JAMA. 2024. PMID: 38227044 No abstract available.
-
US Breast Cancer Mortality.JAMA. 2024 May 21;331(19):1679. doi: 10.1001/jama.2024.5479. JAMA. 2024. PMID: 38662381 No abstract available.
References
-
- Cancer stat facts: female breast cancer. National Cancer Institute. Accessed September 20, 2022. https://seer.cancer.gov/statfacts/html/breast.html
-
- Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Mortality all COD, aggregated with state, total U.S. (1969-2019) <Katrina/Rita Population Adjustment>. Accessed January 31, 2023. https://seer.cancer.gov/data/
-
- Clinical trial search page. National Library of Medicine. Accessed July 7, 2023. https://clinicaltrials.gov/search?cond=breast%20cancer&term=phase%203
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

