Impact of Istradefylline on Levodopa Dose Escalation in Parkinson's Disease: ISTRA ADJUST PD Study, a Multicenter, Open-Label, Randomized, Parallel-Group Controlled Study
- PMID: 38227133
- PMCID: PMC10951171
- DOI: 10.1007/s40120-023-00574-6
Impact of Istradefylline on Levodopa Dose Escalation in Parkinson's Disease: ISTRA ADJUST PD Study, a Multicenter, Open-Label, Randomized, Parallel-Group Controlled Study
Abstract
Introduction: A higher levodopa dose is a risk factor for motor complications in Parkinson's disease (PD). Istradefylline (IST) is used as adjunctive treatment to levodopa in PD patients with off episodes, but its impact on levodopa dose titration remains unclear. The objective of this study was to investigate the effect of IST on levodopa dose escalation in PD patients with wearing-off.
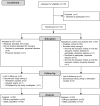
Methods: This was a multicenter, open-label, randomized, parallel-group controlled study (ISTRA ADJUST PD) in which PD patients experiencing wearing-off (n = 114) who were receiving levodopa 300-400 mg/day were randomized to receive IST or no IST (control). Levodopa dose was escalated according to clinical severity. The primary endpoint was cumulative additional levodopa dose, and secondary endpoints were changes in symptom rating scales, motor activity determined by a wearable device, and safety outcomes.
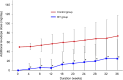
Results: The cumulative additional levodopa dose throughout 37 weeks and dose increase over 36 weeks were significantly lower in the IST group than in the control group (both p < 0.0001). The Movement Disorder Society Unified Parkinson's Disease Rating Scale Part I and device-evaluated motor activities improved significantly from baseline to 36 weeks in the IST group only (all p < 0.05). Other secondary endpoints were comparable between the groups. Adverse drug reactions (ADRs) occurred in 28.8% and 13.2% of patients in the IST and control groups, respectively, with no serious ADRs in either group.
Conclusion: IST treatment reduced levodopa dose escalation in PD patients, resulting in less cumulative levodopa use. Adjunctive IST may improve motor function more objectively than increased levodopa dose in patients with PD.
Trial registration: Japan Registry of Clinical Trials: jRCTs031180248.
Keywords: Adenosine A2A receptor antagonist; Istradefylline; Levodopa; Levodopa dose; Parkinson’s disease.
© 2024. The Author(s).
Conflict of interest statement
Taku Hatano reports receiving grants from the Setsuro Fujii Memorial, the Osaka Foundation for Promotion of Fundamental Medical Research, JSPS KAKENHI (under grant number 21K07424), Japan Agency for Medical Research and Development (grant number 20dm0107156, 21wm0425015, and 21dk0207055); speaker’s honoraria from Sumitomo Pharma Co., Ltd., Takeda Pharmaceutical Co. Ltd., Novartis Pharma K.K., Sanofi K.K., Eisai Co. Ltd. and Otsuka Pharmaceutical Co., Ltd. during the conduct of the study. Taku Hatano also reports receiving grants and speaker’s honoraria from Kyowa Kirin Co., Ltd. during the conduct of the study. Renpei Sengoku reports no relevant disclosures. Renpei Sengoku, Hiroshi Nagayama, Naotake Yanagisawa, Keisuke Suzuki, and Hiroo Terashi report grants and consultation fees from Kyowa Kirin Co., Ltd. during the conduct of the study. Asako Yoritaka received speaker’s honoraria from Kyowa Kirin Co., Ltd. during the conduct of the study. Noriko Nishikawa, Kyoichi Nomura, Norihito Yoshida, Morinobu Seki, Miho Kawabe Matsukawa, Shigeki Hirano, Hidetomo Murakami, Hideto Joki, Tsuyoshi Uchiyama, Kotaro Ogaki, Jiro Fukae, Kazushi Takahashi, and Toshimasa Yamamoto report grants from Kyowa Kirin Co., Ltd. during the conduct of the study. Hiroshi Nagayama reports no relevant disclosures. Naotake Yanagisawa reports no relevant disclosures. Asako Yoritaka received lecture fees from Sumitomo Pharma Co., Ltd., Takeda Pharmaceutical Co., Ltd., Eisai Co., Ltd. and Kyowa Kirin Co., Ltd. Keisuke Suzuki reports receiving speaker honoraria from Kyowa Kirin Co. Ltd, Otsuka Pharmaceutical, Co. Ltd, Sumitomo Pharma Co. Ltd, Takeda Pharmaceuticals Co. Ltd, Eisai Co. Ltd, and Novartis Pharma K.K. Noriko Nishikawa reports no relevant disclosures. Yohei Mukai reports no relevant disclosures. Yohei Mukai reports receiving honoraria fee for lectures from Kyowa Kirin Co., Ltd. during the conduct of the study. Kyoichi Nomura reports no relevant disclosures. Norihito Yoshida reports no relevant disclosures. Morinobu Seki received a Takeda Japan Medical Affairs Funded Research Grant 2018, and grants from Kanae Foundation for the Promotion of Medical Science, outside the submitted work. Miho Kawabe Matsukawa reports no relevant disclosures. Hiroo Terashi reports no relevant disclosures. Katsuo Kimura reports personal fees from Medtronic Japan Co., Ltd., Boston Scientific Corporation, Ono Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., AbbVie GK, Eisai Co., Ltd., Takeda Pharmaceutical Co., Ltd., and Sumitomo Pharma Co., Ltd., and grants from Novartis AG, outside the submitted work. Katsuo Kimura reports grants and lecture fees from Kyowa Kirin Co., Ltd. during the conduct of the study. Jun Tashiro reports grants and non-financial support from Kyowa Kirin Co., Ltd. during the conduct of the study. Hideki Shimura reports grants and lecture fees from Kyowa Kirin Co., Ltd. during the conduct of the study. Yoshio Tsuboi, Kenichi Kaida, Ryoko Ihara, Kazutomi Kanemaru, and Osamu Kano report no relevant disclosures during the conduct of the study. Jun Tashiro reports grants and non-financial support from CSL Behring K.K. and personal fees and non-financial support from Kyowa Kirin Co., Ltd., Takeda Pharmaceutical Co., Ltd., Sumitomo Pharma Co., Ltd., FP Pharmaceutical Corporation, Ono Pharmaceutical Co., Ltd., Eisai Co., Ltd., and AbbVie GK outside the submitted work. Shigeki Hirano reports grants from Eli Lilly Japan K.K., and grants and lecture fees from Nihon Medi-Physics Co., Ltd. outside the submitted work. Hidetomo Murakami reports no relevant disclosures. Hideto Joki reports no relevant disclosures. Tsuyoshi Uchiyama reports no relevant disclosures. Hideki Shimura reports no relevant disclosures. Kotaro Ogaki reports receiving grants from JSPS KAKENHI (under grant number 19K17047), speakers honoraria from Sumitomo Pharma Co., Ltd, Takeda Pharmaceutical Co. Ltd., Kyowa Kirin Co. Ltd., Otsuka Pharmaceutical Co. Ltd., Ono Pharmaceutical Co. Ltd., FP Pharmaceutical Co. Ltd., Mochida Pharmaceutical Co. Ltd., Daiichi Sankyo Co. Ltd, and Eli Lilly Japan K.K. Jiro Fukae reports no relevant disclosures. Yoshio Tsuboi received personal fees from Eisai Co., Ltd., Takeda Pharmaceutical Co., Ltd., Novartis Pharma K.K., Sumitomo Pharma Co., Ltd., AbbVie GK, and Otsuka Pharmaceutical Co., Ltd., and Kyowa Kirin Co., Ltd., outside the submitted work; and were supported by a grant from Nipro Corporation. Kazushi Takahashi reports no relevant disclosures. Toshimasa Yamamoto reports no relevant disclosures. Kenichi Kaida reports no relevant disclosures. Ryoko Ihara reports no relevant disclosures. Kazutomi Kanemaru reports no relevant disclosures. Osamu Kano reports honoraria from AbbVie GK, Alexion Pharmaceuticals, Inc., Biogen Japan Ltd., Biogen Inc., Chugai Pharmaceutical Co., Ltd., Sumitomo Pharma Co., Ltd., Eisai Co., Ltd, Kyowa Kirin Co., Ltd., Mitsubishi Tanabe Pharma Co., Mochida Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., and scholarship grants from Eisai Co., Ltd., and Otsuka Pharmaceutical Co., Ltd., and writing fees from Mitsubishi Tanabe Pharma Co., outside the submitted work, and grants for commissioned work from Alexion Pharmaceuticals, Inc., and Mitsubishi Tanabe Pharma Co.
Figures

References
LinkOut - more resources
Full Text Sources

