Myeloid Zfhx3 deficiency protects against hypercapnia-induced suppression of host defense against influenza A virus
- PMID: 38227369
- PMCID: PMC11143927
- DOI: 10.1172/jci.insight.170316
Myeloid Zfhx3 deficiency protects against hypercapnia-induced suppression of host defense against influenza A virus
Abstract
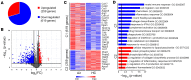
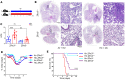
Hypercapnia, elevation of the partial pressure of CO2 in blood and tissues, is a risk factor for mortality in patients with severe acute and chronic lung diseases. We previously showed that hypercapnia inhibits multiple macrophage and neutrophil antimicrobial functions and that elevated CO2 increases the mortality of bacterial and viral pneumonia in mice. Here, we show that normoxic hypercapnia downregulates innate immune and antiviral gene programs in alveolar macrophages (AMØs). We also show that zinc finger homeobox 3 (Zfhx3) - a mammalian ortholog of zfh2, which mediates hypercapnic immune suppression in Drosophila - is expressed in mouse and human macrophages. Deletion of Zfhx3 in the myeloid lineage blocked the suppressive effect of hypercapnia on immune gene expression in AMØs and decreased viral replication, inflammatory lung injury, and mortality in hypercapnic mice infected with influenza A virus. To our knowledge, our results establish Zfhx3 as the first known mammalian mediator of CO2 effects on immune gene expression and lay the basis for future studies to identify therapeutic targets to interrupt hypercapnic immunosuppression in patients with advanced lung disease.
Keywords: Immunology; Influenza; Innate immunity; Macrophages; Pulmonology.
Conflict of interest statement
Figures



Update of
-
Myeloid Zfhx3 Deficiency Protects Against Hypercapnia-induced Suppression of Host Defense Against Influenza A Virus.bioRxiv [Preprint]. 2023 Mar 1:2023.02.28.530480. doi: 10.1101/2023.02.28.530480. bioRxiv. 2023. Update in: JCI Insight. 2024 Jan 16;9(4):e170316. doi: 10.1172/jci.insight.170316. PMID: 36909510 Free PMC article. Updated. Preprint.
Similar articles
-
Hypercapnia Increases Influenza A Virus Infection of Bronchial Epithelial Cells by Augmenting Cellular Cholesterol via mTOR and Akt.Int J Mol Sci. 2025 Apr 26;26(9):4133. doi: 10.3390/ijms26094133. Int J Mol Sci. 2025. PMID: 40362373 Free PMC article.
-
Myeloid Zfhx3 Deficiency Protects Against Hypercapnia-induced Suppression of Host Defense Against Influenza A Virus.bioRxiv [Preprint]. 2023 Mar 1:2023.02.28.530480. doi: 10.1101/2023.02.28.530480. bioRxiv. 2023. Update in: JCI Insight. 2024 Jan 16;9(4):e170316. doi: 10.1172/jci.insight.170316. PMID: 36909510 Free PMC article. Updated. Preprint.
-
Damage sensing through TLR9 regulates inflammatory and antiviral responses during influenza infection.Mucosal Immunol. 2025 Jun;18(3):537-548. doi: 10.1016/j.mucimm.2025.01.008. Epub 2025 Jan 28. Mucosal Immunol. 2025. PMID: 39884393 Free PMC article.
-
Physical interventions to interrupt or reduce the spread of respiratory viruses.Cochrane Database Syst Rev. 2023 Jan 30;1(1):CD006207. doi: 10.1002/14651858.CD006207.pub6. Cochrane Database Syst Rev. 2023. PMID: 36715243 Free PMC article.
-
Permissive hypercapnia for the prevention of morbidity and mortality in mechanically ventilated newborn infants.Cochrane Database Syst Rev. 2001;2001(2):CD002061. doi: 10.1002/14651858.CD002061. Cochrane Database Syst Rev. 2001. PMID: 11406029 Free PMC article.
Cited by
-
KLF17 is an important regulatory component of the transcriptomic response of Atlantic salmon macrophages to Piscirickettsia salmonis infection.Front Immunol. 2023 Dec 14;14:1264599. doi: 10.3389/fimmu.2023.1264599. eCollection 2023. Front Immunol. 2023. PMID: 38162669 Free PMC article.
-
Insight into noncanonical small noncoding RNAs in Influenza A virus infection.Virus Res. 2024 Dec;350:199474. doi: 10.1016/j.virusres.2024.199474. Epub 2024 Sep 27. Virus Res. 2024. PMID: 39326700 Free PMC article.
-
Hypercapnia Increases Influenza A Virus Infection of Bronchial Epithelial Cells by Augmenting Cellular Cholesterol via mTOR and Akt.Int J Mol Sci. 2025 Apr 26;26(9):4133. doi: 10.3390/ijms26094133. Int J Mol Sci. 2025. PMID: 40362373 Free PMC article.
-
Hypercapnia increases ACE2 expression and pseudo-SARS-CoV-2 entry in bronchial epithelial cells by augmenting cellular cholesterol.Front Immunol. 2023 Oct 12;14:1251120. doi: 10.3389/fimmu.2023.1251120. eCollection 2023. Front Immunol. 2023. PMID: 37901225 Free PMC article.
-
Rapamycin improves satellite cells' autophagy and muscle regeneration during hypercapnia.JCI Insight. 2025 Jan 9;10(1):e182842. doi: 10.1172/jci.insight.182842. JCI Insight. 2025. PMID: 39589836 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

