A membrane-associated phosphoswitch in Rad controls adrenergic regulation of cardiac calcium channels
- PMID: 38227371
- PMCID: PMC10904049
- DOI: 10.1172/JCI176943
A membrane-associated phosphoswitch in Rad controls adrenergic regulation of cardiac calcium channels
Abstract
The ability to fight or flee from a threat relies on an acute adrenergic surge that augments cardiac output, which is dependent on increased cardiac contractility and heart rate. This cardiac response depends on β-adrenergic-initiated reversal of the small RGK G protein Rad-mediated inhibition of voltage-gated calcium channels (CaV) acting through the Cavβ subunit. Here, we investigate how Rad couples phosphorylation to augmented Ca2+ influx and increased cardiac contraction. We show that reversal required phosphorylation of Ser272 and Ser300 within Rad's polybasic, hydrophobic C-terminal domain (CTD). Phosphorylation of Ser25 and Ser38 in Rad's N-terminal domain (NTD) alone was ineffective. Phosphorylation of Ser272 and Ser300 or the addition of 4 Asp residues to the CTD reduced Rad's association with the negatively charged, cytoplasmic plasmalemmal surface and with CaVβ, even in the absence of CaVα, measured here by FRET. Addition of a posttranslationally prenylated CAAX motif to Rad's C-terminus, which constitutively tethers Rad to the membrane, prevented the physiological and biochemical effects of both phosphorylation and Asp substitution. Thus, dissociation of Rad from the sarcolemma, and consequently from CaVβ, is sufficient for sympathetic upregulation of Ca2+ currents.
Keywords: Calcium channels; Cardiology; Cardiovascular disease; Excitation contraction coupling.
Figures






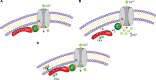
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

