C3aR-initiated signaling is a critical mechanism of podocyte injury in membranous nephropathy
- PMID: 38227377
- PMCID: PMC11143932
- DOI: 10.1172/jci.insight.172976
C3aR-initiated signaling is a critical mechanism of podocyte injury in membranous nephropathy
Abstract
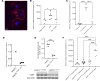
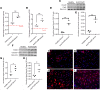
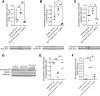
The deposition of antipodocyte autoantibodies in the glomerular subepithelial space induces primary membranous nephropathy (MN), the leading cause of nephrotic syndrome worldwide. Taking advantage of the glomerulus-on-a-chip system, we modeled human primary MN induced by anti-PLA2R antibodies. Here we show that exposure of primary human podocytes expressing PLA2R to MN serum results in IgG deposition and complement activation on their surface, leading to loss of the chip permselectivity to albumin. C3a receptor (C3aR) antagonists as well as C3AR gene silencing in podocytes reduced oxidative stress induced by MN serum and prevented albumin leakage. In contrast, inhibition of the formation of the membrane-attack-complex (MAC), previously thought to play a major role in MN pathogenesis, did not affect permselectivity to albumin. In addition, treatment with a C3aR antagonist effectively prevented proteinuria in a mouse model of MN, substantiating the chip findings. In conclusion, using a combination of pathophysiologically relevant in vitro and in vivo models, we established that C3a/C3aR signaling plays a critical role in complement-mediated MN pathogenesis, indicating an alternative therapeutic target for MN.
Keywords: Chronic kidney disease; Complement; Nephrology.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

