Reversible synaptic adaptations in a subpopulation of murine hippocampal neurons following early-life seizures
- PMID: 38227384
- PMCID: PMC10904056
- DOI: 10.1172/JCI175167
Reversible synaptic adaptations in a subpopulation of murine hippocampal neurons following early-life seizures
Abstract
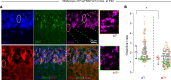
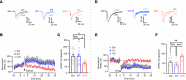
Early-life seizures (ELSs) can cause permanent cognitive deficits and network hyperexcitability, but it is unclear whether ELSs induce persistent changes in specific neuronal populations and whether these changes can be targeted to mitigate network dysfunction. We used the targeted recombination of activated populations (TRAP) approach to genetically label neurons activated by kainate-induced ELSs in immature mice. The ELS-TRAPed neurons were mainly found in hippocampal CA1, remained uniquely susceptible to reactivation by later-life seizures, and displayed sustained enhancement in α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor-mediated (AMPAR-mediated) excitatory synaptic transmission and inward rectification. ELS-TRAPed neurons, but not non-TRAPed surrounding neurons, exhibited enduring decreases in Gria2 mRNA, responsible for encoding the GluA2 subunit of the AMPARs. This was paralleled by decreased synaptic GluA2 protein expression and heightened phosphorylated GluA2 at Ser880 in dendrites, indicative of GluA2 internalization. Consistent with increased GluA2-lacking AMPARs, ELS-TRAPed neurons showed premature silent synapse depletion, impaired long-term potentiation, and impaired long-term depression. In vivo postseizure treatment with IEM-1460, an inhibitor of GluA2-lacking AMPARs, markedly mitigated ELS-induced changes in TRAPed neurons. These findings show that enduring modifications of AMPARs occur in a subpopulation of ELS-activated neurons, contributing to synaptic dysplasticity and network hyperexcitability, but are reversible with early IEM-1460 intervention.
Keywords: Epilepsy; Mouse models; Neuroscience; Seizures.
Conflict of interest statement
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

