How do neurons age? A focused review on the aging of the microtubular cytoskeleton
- PMID: 38227514
- PMCID: PMC11040321
- DOI: 10.4103/1673-5374.390974
How do neurons age? A focused review on the aging of the microtubular cytoskeleton
Abstract
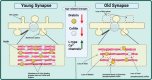
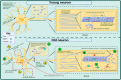
Aging is the leading risk factor for Alzheimer's disease and other neurodegenerative diseases. We now understand that a breakdown in the neuronal cytoskeleton, mainly underpinned by protein modifications leading to the destabilization of microtubules, is central to the pathogenesis of Alzheimer's disease. This is accompanied by morphological defects across the somatodendritic compartment, axon, and synapse. However, knowledge of what occurs to the microtubule cytoskeleton and morphology of the neuron during physiological aging is comparatively poor. Several recent studies have suggested that there is an age-related increase in the phosphorylation of the key microtubule stabilizing protein tau, a modification, which is known to destabilize the cytoskeleton in Alzheimer's disease. This indicates that the cytoskeleton and potentially other neuronal structures reliant on the cytoskeleton become functionally compromised during normal physiological aging. The current literature shows age-related reductions in synaptic spine density and shifts in synaptic spine conformation which might explain age-related synaptic functional deficits. However, knowledge of what occurs to the microtubular and actin cytoskeleton, with increasing age is extremely limited. When considering the somatodendritic compartment, a regression in dendrites and loss of dendritic length and volume is reported whilst a reduction in soma volume/size is often seen. However, research into cytoskeletal change is limited to a handful of studies demonstrating reductions in and mislocalizations of microtubule-associated proteins with just one study directly exploring the integrity of the microtubules. In the axon, an increase in axonal diameter and age-related appearance of swellings is reported but like the dendrites, just one study investigates the microtubules directly with others reporting loss or mislocalization of microtubule-associated proteins. Though these are the general trends reported, there are clear disparities between model organisms and brain regions that are worthy of further investigation. Additionally, longitudinal studies of neuronal/cytoskeletal aging should also investigate whether these age-related changes contribute not just to vulnerability to disease but also to the decline in nervous system function and behavioral output that all organisms experience. This will highlight the utility, if any, of cytoskeletal fortification for the promotion of healthy neuronal aging and potential protection against age-related neurodegenerative disease. This review seeks to summarize what is currently known about the physiological aging of the neuron and microtubular cytoskeleton in the hope of uncovering mechanisms underpinning age-related risk to disease.
Copyright © 2024 Copyright: © 2024 Neural Regeneration Research.
Conflict of interest statement
Figures
Similar articles
-
The neuronal cytoskeleton as a potential therapeutical target in neurodegenerative diseases and schizophrenia.Curr Drug Targets CNS Neurol Disord. 2004 Dec;3(6):515-33. doi: 10.2174/1568007043336761. Curr Drug Targets CNS Neurol Disord. 2004. PMID: 15581421 Review.
-
Neuronal ageing is promoted by the decay of the microtubule cytoskeleton.PLoS Biol. 2024 Mar 13;22(3):e3002504. doi: 10.1371/journal.pbio.3002504. eCollection 2024 Mar. PLoS Biol. 2024. PMID: 38478582 Free PMC article.
-
Investigation of Low Dose Cabazitaxel Potential as Microtubule Stabilizer in Experimental Model of Alzheimer's Disease: Restoring Neuronal Cytoskeleton.Curr Alzheimer Res. 2020;17(7):601-615. doi: 10.2174/1567205017666201007120112. Curr Alzheimer Res. 2020. PMID: 33030130
-
Amyloid beta: a putative intra-spinal microtubule-depolymerizer to induce synapse-loss or dentritic spine shortening in Alzheimer's disease.Ital J Anat Embryol. 2009 Apr-Sep;114(2-3):109-20. Ital J Anat Embryol. 2009. PMID: 20198823 Review.
-
Phosphorylation of microtubule-associated protein 2 (MAP2) and its relevance for the regulation of the neuronal cytoskeleton function.Prog Neurobiol. 2000 Jun;61(2):133-68. doi: 10.1016/s0301-0082(99)00046-5. Prog Neurobiol. 2000. PMID: 10704996 Review.
Cited by
-
Mind-body training outperforms other physical activities in reducing frailty and enhancing quality of life in older adults: a network meta-analysis.Front Public Health. 2025 Jul 14;13:1578791. doi: 10.3389/fpubh.2025.1578791. eCollection 2025. Front Public Health. 2025. PMID: 40726938 Free PMC article.
-
Relationship between Hypoxia and Hypercapnia Tolerance and Life Expectancy.Int J Mol Sci. 2024 Jun 13;25(12):6512. doi: 10.3390/ijms25126512. Int J Mol Sci. 2024. PMID: 38928217 Free PMC article. Review.
-
The causal effects of intelligence and fluid intelligence on Parkinson's disease: a Mendelian randomization study.Front Aging Neurosci. 2024 May 23;16:1388795. doi: 10.3389/fnagi.2024.1388795. eCollection 2024. Front Aging Neurosci. 2024. PMID: 38846742 Free PMC article.
-
Stiffness-tunable biomaterials provide a good extracellular matrix environment for axon growth and regeneration.Neural Regen Res. 2025 May 1;20(5):1364-1376. doi: 10.4103/NRR.NRR-D-23-01874. Epub 2024 May 13. Neural Regen Res. 2025. PMID: 39075897 Free PMC article.
References
-
- Ackerman S. Washington (DC): National Academies Press (US); 1992. Discovering the brain. - PubMed
-
- Aguilar-Hernández L, Vázquez-Hernández AJ, de-Lima-Mar DF, Vázquez-Roque RA, Tendilla-Beltrán H, Flores G. Memory and dendritic spines loss, and dynamic dendritic spines changes are age-dependent in the rat. J Chem Neuroanat. 2020;110:101858. - PubMed
LinkOut - more resources
Full Text Sources

