Therapeutic advances in neural regeneration for Huntington's disease
- PMID: 38227527
- PMCID: PMC11040295
- DOI: 10.4103/1673-5374.390969
Therapeutic advances in neural regeneration for Huntington's disease
Abstract
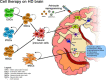
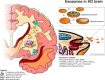
Huntington's disease is a neurodegenerative disease caused by the expansion mutation of a cytosine-adenine-guanine triplet in the exon 1 of the HTT gene which is responsible for the production of the huntingtin (Htt) protein. In physiological conditions, Htt is involved in many cellular processes such as cell signaling, transcriptional regulation, energy metabolism regulation, DNA maintenance, axonal trafficking, and antiapoptotic activity. When the genetic alteration is present, the production of a mutant version of Htt (mHtt) occurs, which is characterized by a plethora of pathogenic activities that, finally, lead to cell death. Among all the cells in which mHtt exerts its dangerous activity, the GABAergic Medium Spiny Neurons seem to be the most affected by the mHtt-induced excitotoxicity both in the cortex and in the striatum. However, as the neurodegeneration proceeds ahead the neuronal loss grows also in other brain areas such as the cerebellum, hypothalamus, thalamus, subthalamic nucleus, globus pallidus, and substantia nigra, determining the variety of symptoms that characterize Huntington's disease. From a clinical point of view, Huntington's disease is characterized by a wide spectrum of symptoms spanning from motor impairment to cognitive disorders and dementia. Huntington's disease shows a prevalence of around 3.92 cases every 100,000 worldwide and an incidence of 0.48 new cases every 100,000/year. To date, there is no available cure for Huntington's disease. Several treatments have been developed so far, aiming to reduce the severity of one or more symptoms to slow down the inexorable decline caused by the disease. In this context, the search for reliable strategies to target the different aspects of Huntington's disease become of the utmost interest. In recent years, a variety of studies demonstrated the detrimental role of neuronal loss in Huntington's disease condition highlighting how the replacement of lost cells would be a reasonable strategy to overcome the neurodegeneration. In this view, numerous have been the attempts in several preclinical models of Huntington's disease to evaluate the feasibility of invasive and non-invasive approaches. Thus, the aim of this review is to offer an overview of the most appealing approaches spanning from stem cell-based cell therapy to extracellular vesicles such as exosomes in light of promoting neurogenesis, discussing the results obtained so far, their limits and the future perspectives regarding the neural regeneration in the context of Huntington's disease.
Copyright © 2024 Copyright: © 2024 Neural Regeneration Research.
Conflict of interest statement
Figures
Similar articles
-
Downregulation of glial genes involved in synaptic function mitigates Huntington's disease pathogenesis.Elife. 2021 Apr 19;10:e64564. doi: 10.7554/eLife.64564. Elife. 2021. PMID: 33871358 Free PMC article.
-
Therapeutic reversal of Huntington's disease by in vivo self-assembled siRNAs.Brain. 2021 Dec 16;144(11):3421-3435. doi: 10.1093/brain/awab354. Brain. 2021. PMID: 34918046 Free PMC article.
-
Amelioration of Huntington's disease phenotype in astrocytes derived from iPSC-derived neural progenitor cells of Huntington's disease monkeys.PLoS One. 2019 Mar 21;14(3):e0214156. doi: 10.1371/journal.pone.0214156. eCollection 2019. PLoS One. 2019. PMID: 30897183 Free PMC article.
-
Therapeutic Advances for Huntington's Disease.Brain Sci. 2020 Jan 12;10(1):43. doi: 10.3390/brainsci10010043. Brain Sci. 2020. PMID: 31940909 Free PMC article. Review.
-
Protective Effects of Antioxidants in Huntington's Disease: an Extensive Review.Neurotox Res. 2019 Apr;35(3):739-774. doi: 10.1007/s12640-018-9989-9. Epub 2019 Jan 11. Neurotox Res. 2019. PMID: 30632085 Review.
Cited by
-
Increased excitatory amino acid transporter 2 levels in basolateral amygdala astrocytes mediate chronic stress-induced anxiety-like behavior.Neural Regen Res. 2025 Jun 1;20(6):1721-1734. doi: 10.4103/NRR.NRR-D-23-01411. Epub 2024 Apr 16. Neural Regen Res. 2025. PMID: 39104111 Free PMC article.
-
Calcium bridges built by mitochondria-associated endoplasmic reticulum membranes: potential targets for neural repair in neurological diseases.Neural Regen Res. 2025 Dec 1;20(12):3349-3369. doi: 10.4103/NRR.NRR-D-24-00630. Epub 2024 Nov 13. Neural Regen Res. 2025. PMID: 39589178 Free PMC article.
-
Advances in gene and cellular therapeutic approaches for Huntington's disease.Protein Cell. 2025 May 28;16(5):307-337. doi: 10.1093/procel/pwae042. Protein Cell. 2025. PMID: 39121016 Free PMC article. Review.
-
Cerebellar microglia: On the edge between neuroinflammation and neuroregulation.Neural Regen Res. 2026 Jan 1;21(1):156-172. doi: 10.4103/NRR.NRR-D-24-00550. Epub 2024 Oct 22. Neural Regen Res. 2026. PMID: 40489344 Free PMC article.
-
Cerebellum in neurodegenerative diseases: Advances, challenges, and prospects.iScience. 2024 Oct 18;27(11):111194. doi: 10.1016/j.isci.2024.111194. eCollection 2024 Nov 15. iScience. 2024. PMID: 39555407 Free PMC article. Review.
References
-
- Abdollah Zadegan S, Coco HM, Reddy KS, Anderson KM, Teixeira AL, Stimming EF. Frequency and pathophysiology of apathy in Huntington disease: a systematic review and meta-analysis. J Neuropsychiatry Clin Neurosci. 2023;35:121–132. - PubMed
LinkOut - more resources
Full Text Sources

