Metformin alleviates spinal cord injury by inhibiting nerve cell ferroptosis through upregulation of heme oxygenase-1 expression
- PMID: 38227534
- PMCID: PMC11040287
- DOI: 10.4103/1673-5374.390960
Metformin alleviates spinal cord injury by inhibiting nerve cell ferroptosis through upregulation of heme oxygenase-1 expression
Abstract
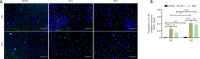
JOURNAL/nrgr/04.03/01300535-202409000-00037/figure1/v/2024-01-16T170235Z/r/image-tiff Previous studies have reported upregulation of heme oxygenase-1 in different central nervous system injury models. Heme oxygenase-1 plays a critical anti-inflammatory role and is essential for regulating cellular redox homeostasis. Metformin is a classic drug used to treat type 2 diabetes that can inhibit ferroptosis. Previous studies have shown that, when used to treat cardiovascular and digestive system diseases, metformin can also upregulate heme oxygenase-1 expression. Therefore, we hypothesized that heme oxygenase-1 plays a significant role in mediating the beneficial effects of metformin on neuronal ferroptosis after spinal cord injury. To test this, we first performed a bioinformatics analysis based on the GEO database and found that heme oxygenase-1 was upregulated in the lesion of rats with spinal cord injury. Next, we confirmed this finding in a rat model of T9 spinal cord compression injury that exhibited spinal cord nerve cell ferroptosis. Continuous intraperitoneal injection of metformin for 14 days was found to both upregulate heme oxygenase-1 expression and reduce neuronal ferroptosis in rats with spinal cord injury. Subsequently, we used a lentivirus vector to knock down heme oxygenase-1 expression in the spinal cord, and found that this significantly reduced the effect of metformin on ferroptosis after spinal cord injury. Taken together, these findings suggest that metformin inhibits neuronal ferroptosis after spinal cord injury, and that this effect is partially dependent on upregulation of heme oxygenase-1.
Copyright © 2024 Copyright: © 2024 Neural Regeneration Research.
Conflict of interest statement
Figures



Similar articles
-
Biochanin A attenuates spinal cord injury in rats during early stages by inhibiting oxidative stress and inflammasome activation.Neural Regen Res. 2024 Sep 1;19(9):2050-2056. doi: 10.4103/1673-5374.390953. Epub 2023 Dec 15. Neural Regen Res. 2024. PMID: 38227535 Free PMC article.
-
USP7 regulates HMOX-1 via deubiquitination to suppress ferroptosis and ameliorate spinal cord injury in rats.Neurochem Int. 2023 Sep;168:105554. doi: 10.1016/j.neuint.2023.105554. Epub 2023 May 29. Neurochem Int. 2023. PMID: 37257587
-
Protein arginine methyltransferase 8 regulates ferroptosis and macrophage polarization in spinal cord injury via glial cell-derived neurotrophic factor.CNS Neurosci Ther. 2023 Aug;29(8):2145-2161. doi: 10.1111/cns.14162. Epub 2023 Mar 13. CNS Neurosci Ther. 2023. PMID: 36914965 Free PMC article.
-
Ferroptosis: a critical player and potential therapeutic target in traumatic brain injury and spinal cord injury.Neural Regen Res. 2023 Mar;18(3):506-512. doi: 10.4103/1673-5374.350187. Neural Regen Res. 2023. PMID: 36018155 Free PMC article. Review.
-
Mesenchymal stem cells, extracellular vesicles, and transcranial magnetic stimulation for ferroptosis after spinal cord injury.Neural Regen Res. 2023 Sep;18(9):1861-1868. doi: 10.4103/1673-5374.367838. Neural Regen Res. 2023. PMID: 36926700 Free PMC article. Review.
Cited by
-
Glycosylated lysosomal membrane protein promotes tissue repair after spinal cord injury by reducing iron deposition and ferroptosis in microglia.Sci Rep. 2025 Jan 22;15(1):2867. doi: 10.1038/s41598-025-86991-z. Sci Rep. 2025. PMID: 39843796 Free PMC article.
-
Microglia overexpressing brain-derived neurotrophic factor promote vascular repair and functional recovery in mice after spinal cord injury.Neural Regen Res. 2026 Jan 1;21(1):365-376. doi: 10.4103/NRR.NRR-D-24-00381. Epub 2024 Oct 22. Neural Regen Res. 2026. PMID: 39435607 Free PMC article.
-
Metformin enhances endogenous neural stem cells proliferation, neuronal differentiation, and inhibits ferroptosis through activating AMPK pathway after spinal cord injury.J Transl Med. 2024 Aug 5;22(1):723. doi: 10.1186/s12967-024-05436-9. J Transl Med. 2024. PMID: 39103875 Free PMC article.
-
Iron homeostasis and ferroptosis in human diseases: mechanisms and therapeutic prospects.Signal Transduct Target Ther. 2024 Oct 14;9(1):271. doi: 10.1038/s41392-024-01969-z. Signal Transduct Target Ther. 2024. PMID: 39396974 Free PMC article. Review.
-
Recombinant chitinase-3-like protein 1 alleviates learning and memory impairments via M2 microglia polarization in postoperative cognitive dysfunction mice.Neural Regen Res. 2025 Sep 1;20(9):2727-2736. doi: 10.4103/NRR.NRR-D-23-01233. Epub 2024 Jul 10. Neural Regen Res. 2025. PMID: 38993135 Free PMC article.
References
-
- Adegeest CY, van Gent JAN, Stolwijk-Swüste JM, Post MWM, Vandertop WP, Öner FC, Peul WC, Wengel PVT. Influence of severity and level of injury on the occurrence of complications during the subacute and chronic stage of traumatic spinal cord injury: a systematic review. J Neurosurg Spine. 2022;36:632–652. - PubMed
-
- Bin S, Xin L, Lin Z, Jinhua Z, Rui G, Xiang Z. Targeting miR-10a-5p/IL-6R axis for reducing IL-6-induced cartilage cell ferroptosis. Exp Mol Pathol. 2021;118:104570. - PubMed
-
- Cai X, Hua S, Deng J, Du Z, Zhang D, Liu Z, Khan NU, Zhou M, Chen Z. Astaxanthin activated the Nrf2/HO-1 pathway to enhance autophagy and inhibit ferroptosis, ameliorating acetaminophen-induced liver injury. ACS Appl Mater Interfaces. 2022;14:42887–42903. - PubMed
-
- Campbell NK, Fitzgerald HK, Dunne A. Regulation of inflammation by the antioxidant haem oxygenase 1. Nat Rev Immunol. 2021;21:411–425. - PubMed
LinkOut - more resources
Full Text Sources

