Clinical manifestations and spermatogenesis outcomes in Chinese patients with congenital hypogonadotropic hypogonadism caused by inherited or de novo FGFR1 mutations
- PMID: 38227553
- PMCID: PMC11280213
- DOI: 10.4103/aja202366
Clinical manifestations and spermatogenesis outcomes in Chinese patients with congenital hypogonadotropic hypogonadism caused by inherited or de novo FGFR1 mutations
Abstract
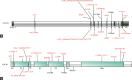
Fibroblast growth factor receptor 1 ( FGFR1 ) mutations are associated with congenital hypogonadotropic hypogonadism (CHH) through inheritance or spontaneous occurrence. We detected FGFR1 mutations in a Chinese cohort of 210 CHH patients at Peking Union Medical College Hospital (Beijing, China) using next-generation and Sanger sequencing. We assessed missense variant pathogenicity using six bioinformatics tools and compared clinical features and treatment outcomes between inherited and de novo mutation groups. Among 19 patients with FGFR1 mutations, three were recurrent, and 16 were novel variants. Sixteen of the novel mutations were likely pathogenic according to the American College of Medical Genetics and Genomics (ACMG) guidelines, with the prevalent P366L variant. The majority of FGFR1 mutations was inherited (57.9%), with frameshift mutations exclusive to the de novo mutation group. The inherited mutation group had a lower incidence of cryptorchidism, short stature, and skeletal deformities. In the inherited mutation group, luteinizing hormone (LH) levels were 0.5 IU l -1 , follicle-stimulating hormone (FSH) levels were 1.0 IU l -1 , and testosterone levels were 1.3 nmol l -1 . In contrast, the de novo group had LH levels of 0.2 IU l -1 , FSH levels of 0.5 IU l -1 , and testosterone levels of 0.9 nmol l -1 , indicating milder hypothalamus-pituitary-gonadal axis (HPGA) functional deficiency in the inherited group. The inherited mutation group showed a tendency toward higher spermatogenesis rates. In conclusion, this study underscores the predominance of inherited FGFR1 mutations and their association with milder HPGA dysfunction compared to de novo mutations, contributing to our understanding of the genetic and clinical aspects of FGFR1 mutations.
Copyright © 2024 Copyright: ©The Author(s)(2024).
Conflict of interest statement
All authors declare no competing interests.
Figures
References
-
- Young J, Xu C, Papadakis GE, Acierno JS, Maione L, et al. Clinical management of congenital hypogonadotropic hypogonadism. Endocr Rev. 2019;40:669–710. - PubMed
-
- Boehm U, Bouloux PM, Dattani MT, de Roux N, Dodé C, et al. Expert consensus document: European Consensus Statement on congenital hypogonadotropic hypogonadism –pathogenesis, diagnosis and treatment. Nat Rev Endocrinol. 2015;11:547–64. - PubMed
-
- Lima Amato LG, Latronico AC, Gontijo Silveira LF. Molecular and genetic aspects of congenital isolated hypogonadotropic hypogonadism. Endocrinol Metab Clin North Am. 2017;46:283–303. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

