Can observational learning reinforce open-label placebo hypoalgesia?
- PMID: 38227574
- PMCID: PMC11190895
- DOI: 10.1097/j.pain.0000000000003161
Can observational learning reinforce open-label placebo hypoalgesia?
Abstract
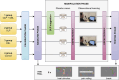
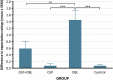
Previous research has indicated that an open-label placebo can reduce pain in both healthy participants and patients with chronic pain. Because nondeceptive placebos seem to be an effective and more ethical alternative to deceptive placebos, optimizing this kind of treatment is essential. Observational learning was previously shown to induce the deceptive placebo effect; therefore, this study aimed to verify its effectiveness in fortifying the open-label placebo effect. Healthy volunteers (N = 117) were randomly assigned to 4 groups: open-label placebo with observational learning (OLP + OBL), open-label placebo (OLP), deceptive placebo with observational learning (OBL), or control group. Participants underwent baseline and testing measurements, during which they self-reported pain induced by heat stimulation. Between assessments, placebo cream was openly administered in the OLP and OLP + OBL groups. The OLP + OBL group next watched a model experiencing hypoalgesia after cream application. In the OBL group, participants received placebo cream with no information about its effect, and then they watched the model. The placebo effect was successfully evoked in all experimental groups (OLP + OBL, OLP, and OBL), which confirms the effectiveness of both open-label and deceptive placebo interventions for pain reduction. However, the hypoalgesic effect was of similar magnitude in the OLP and OLP + OBL groups, which indicates that observation did not contribute to the effect. The results showed that reinforcing the open-label placebo by observational learning may be redundant, but more research is needed to confirm these findings.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the International Association for the Study of Pain.
Conflict of interest statement
The authors have no conflict of interest to declare.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures

References
-
- Bąbel P. The effect of question wording in questionnaire surveys on placebo use in clinical practice. Eval Health Prof 2012;35:447–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

