Synthesis of 15N-Pyridines and Higher Mass Isotopologs via Zincke Imine Intermediates
- PMID: 38227776
- PMCID: PMC11446173
- DOI: 10.1021/jacs.3c12445
Synthesis of 15N-Pyridines and Higher Mass Isotopologs via Zincke Imine Intermediates
Abstract
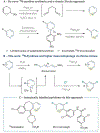
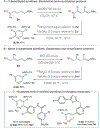
Methods to incorporate stable radioisotopes are integral to pharmaceutical and agrochemical development. However, despite the prevalence of pyridines in candidate compounds, methods to incorporate 15N atoms within their structures are limited. Here, we present a general approach to pyridine 15N-labeling that proceeds via ring-opening to NTf-Zincke imines and then ring-closure with commercially available 15NH4Cl salts. This process functions on a range of substituted pyridines, from simple building block-type compounds to late-stage labeling of complex pharmaceuticals, and 15N-incorporation is >95% in most cases. The reactivity of the Zincke imine intermediates also enables deuteration of the pyridine C3- and C5-positions, resulting in higher mass isotopologs required for LCMS analysis of biological fluids during drug development.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
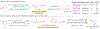
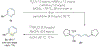
References
-
- Elmore CS; Bragg RA Isotope Chemistry; A Useful Tool In The Drug Discovery Arsenal. Bioorg. Med. Chem. Lett. 2015, 25, 167–171. - PubMed
-
- Dalvie D Recent Advances in the Applications of Radioisotopes in Drug Metabolism, Toxicology and Pharmacokinetics. Curr. Pharm. Des. 2000, 6, 1009–1028. - PubMed
-
- Hesk D; McNamara P Synthesis of Isotopically Labelled Compounds at Schering- Plough, an Historical Perspective. J. Label. Compd. Radiopharm. 2007, 50, 875–887.
-
- Theillet FX In-Cell Structural Biology by NMR: The Benefits of the Atomic Scale. Chem. Rev. 2022, 122, 9497–9570. - PubMed
-
- Lehmann WD A Timeline of Stable Isotopes and Mass Spectrometry in the Life Sciences. Mass. Spectrom. Rev. 2017, 36, 58–85. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

