Lenvatinib Plus Pembrolizumab Versus Sunitinib in First-Line Treatment of Advanced Renal Cell Carcinoma: Final Prespecified Overall Survival Analysis of CLEAR, a Phase III Study
- PMID: 38227898
- PMCID: PMC11095851
- DOI: 10.1200/JCO.23.01569
Lenvatinib Plus Pembrolizumab Versus Sunitinib in First-Line Treatment of Advanced Renal Cell Carcinoma: Final Prespecified Overall Survival Analysis of CLEAR, a Phase III Study
Abstract
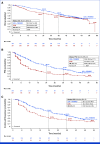
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical trial updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.We present the final prespecified overall survival (OS) analysis of the open-label, phase III CLEAR study in treatment-naïve patients with advanced renal cell carcinoma (aRCC). With an additional follow-up of 23 months from the primary analysis, we report results from the lenvatinib plus pembrolizumab versus sunitinib comparison of CLEAR. Treatment-naïve patients with aRCC were randomly assigned to receive lenvatinib (20 mg orally once daily in 21-day cycles) plus pembrolizumab (200 mg intravenously once every 3 weeks) or sunitinib (50 mg orally once daily [4 weeks on/2 weeks off]). At this data cutoff date (July 31, 2022), the OS hazard ratio (HR) was 0.79 (95% CI, 0.63 to 0.99). The median OS (95% CI) was 53.7 months (95% CI, 48.7 to not estimable [NE]) with lenvatinib plus pembrolizumab versus 54.3 months (95% CI, 40.9 to NE) with sunitinib; 36-month OS rates (95% CI) were 66.4% (95% CI, 61.1 to 71.2) and 60.2% (95% CI, 54.6 to 65.2), respectively. The median progression-free survival (95% CI) was 23.9 months (95% CI, 20.8 to 27.7) with lenvatinib plus pembrolizumab and 9.2 months (95% CI, 6.0 to 11.0) with sunitinib (HR, 0.47 [95% CI, 0.38 to 0.57]). Objective response rate also favored the combination over sunitinib (71.3% v 36.7%; relative risk 1.94 [95% CI, 1.67 to 2.26]). Treatment-emergent adverse events occurred in >90% of patients who received either treatment. In conclusion, lenvatinib plus pembrolizumab achieved consistent, durable benefit with a manageable safety profile in treatment-naïve patients with aRCC.
Trial registration: ClinicalTrials.gov NCT02811861.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures

References
-
- Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384:1289–1300. - PubMed
-
- Lenvima® (lenvatinib) [prescribing information] Nutley, NJ: Eisai; 2023.
-
- Keytruda® (pembrolizumab) [prescribing information] Rahway, NJ: Merck Sharp & Dohme LLC; 2023.
-
- Choueiri TK, Eto M, Motzer R, et al. Lenvatinib plus pembrolizumab versus sunitinib as first-line treatment of patients with advanced renal cell carcinoma (CLEAR): Extended follow-up from the phase 3, randomised, open-label study. Lancet Oncol. 2023;24:228–238. - PubMed
-
- Latimer NR. Treatment switching in oncology trials and the acceptability of adjustment methods. Expert Rev Pharmacoecon Outcomes Res. 2015;15:561–564. - PubMed

