CD8+ Tissue-Resident Memory T Cells: Versatile Guardians of the Tissue
- PMID: 38227907
- PMCID: PMC10794029
- DOI: 10.4049/jimmunol.2300399
CD8+ Tissue-Resident Memory T Cells: Versatile Guardians of the Tissue
Abstract
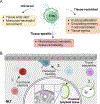
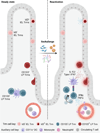
Tissue-resident memory T (Trm) cells are a subset of T cells maintained throughout life within nonlymphoid tissues without significant contribution from circulating memory T cells. CD8+ Trm cells contribute to both tissue surveillance and direct elimination of pathogens through a variety of mechanisms. Reactivation of these Trm cells during infection drives systematic changes within the tissue, including altering the state of the epithelium, activating local immune cells, and contributing to the permissiveness of the tissue for circulating immune cell entry. Trm cells can be further classified by their functional outputs, which can be either subset- or tissue-specific, and include proliferation, tissue egress, and modulation of tissue physiology. These functional outputs of Trm cells are linked to the heterogeneity and plasticity of this population, and uncovering the unique responses of different Trm cell subsets and their role in immunity will allow us to modulate Trm cell responses for optimal control of disease.
Copyright © 2024 by The American Association of Immunologists, Inc.
Figures
Similar articles
-
The evolving role of tissue-resident memory T cells in infections and cancer.Sci Adv. 2022 Aug 19;8(33):eabo5871. doi: 10.1126/sciadv.abo5871. Epub 2022 Aug 17. Sci Adv. 2022. PMID: 35977028 Free PMC article. Review.
-
Genealogy, Dendritic Cell Priming, and Differentiation of Tissue-Resident Memory CD8+ T Cells.Front Immunol. 2018 Jul 31;9:1751. doi: 10.3389/fimmu.2018.01751. eCollection 2018. Front Immunol. 2018. PMID: 30108585 Free PMC article. Review.
-
Insights into phenotypic and functional CD8+ TRM heterogeneity.Immunol Rev. 2023 Jul;316(1):8-22. doi: 10.1111/imr.13218. Epub 2023 May 16. Immunol Rev. 2023. PMID: 37191051 Free PMC article. Review.
-
The Emerging Role of CD8+ Tissue Resident Memory T (TRM) Cells in Antitumor Immunity: A Unique Functional Contribution of the CD103 Integrin.Front Immunol. 2018 Aug 15;9:1904. doi: 10.3389/fimmu.2018.01904. eCollection 2018. Front Immunol. 2018. PMID: 30158938 Free PMC article. Review.
-
Formation of Tissue-Resident CD8+ T-Cell Memory.Cold Spring Harb Perspect Biol. 2021 Aug 2;13(8):a038117. doi: 10.1101/cshperspect.a038117. Cold Spring Harb Perspect Biol. 2021. PMID: 33685935 Free PMC article. Review.
Cited by
-
Aryl hydrocarbon receptor: current perspectives on key signaling partners and immunoregulatory role in inflammatory diseases.Front Immunol. 2024 Aug 15;15:1421346. doi: 10.3389/fimmu.2024.1421346. eCollection 2024. Front Immunol. 2024. PMID: 39211042 Free PMC article. Review.
-
Single-Cell Analysis Reveals Tissue-Specific T Cell Adaptation and Clonal Distribution Across the Human Gut-Liver-Blood Axis.bioRxiv [Preprint]. 2025 Mar 14:2025.03.11.642626. doi: 10.1101/2025.03.11.642626. bioRxiv. 2025. PMID: 40161783 Free PMC article. Preprint.
-
The Reduced Immunogenicity of Zoster Vaccines in CMV-Seropositive Older Adults Correlates with T Cell Imprinting.Vaccines (Basel). 2025 Mar 22;13(4):340. doi: 10.3390/vaccines13040340. Vaccines (Basel). 2025. PMID: 40333195 Free PMC article.
-
Deep profiling deconstructs features associated with memory CD8+ T cell tissue residence.Immunity. 2025 Jan 14;58(1):162-181.e10. doi: 10.1016/j.immuni.2024.11.007. Epub 2024 Dec 20. Immunity. 2025. PMID: 39708817
-
Leveraging tissue-resident memory T cells for non-invasive immune monitoring via microneedle skin patches.medRxiv [Preprint]. 2025 Mar 21:2025.03.17.25324099. doi: 10.1101/2025.03.17.25324099. medRxiv. 2025. PMID: 40166546 Free PMC article. Preprint.
References
-
- Park SL, Zaid A, Hor JL, Christo SN, Prier JE, Davies B, Alexandre YO, Gregory JL, Russell TA, Gebhardt T, Carbone FR, Tscharke DC, Heath WR, Mueller SN, and Mackay LK. 2018. Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nat Immunol 19: 183–191. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

