Synthesis of Boron-Containing Nucleoside Analogs
- PMID: 38227951
- PMCID: PMC10845115
- DOI: 10.1021/acs.joc.3c02179
Synthesis of Boron-Containing Nucleoside Analogs
Abstract
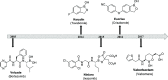
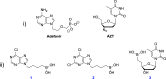
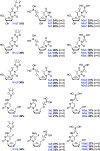
Over the last century, nucleoside-based therapeutics have demonstrated remarkable effectiveness in the treatment of a wide variety of diseases from cancer to HIV. In addition, boron-containing drugs have recently emerged as an exciting and fruitful avenue for medicinal therapies. However, borononucleosides have largely been unexplored in the context of medicinal applications. Herein, we report the synthesis, isolation, and characterization of two novel boron-containing nucleoside compound libraries which may find utility as therapeutic agents. Our synthetic strategy employs efficient one-step substitution reactions between a diverse variety of nucleoside scaffolds and an assortment of n-alkyl potassium trifluoroborate-containing electrophiles. We demonstrated that these alkylation reactions are compatible with cyclic and acyclic nucleoside substrates, as well as increasing alkyl chain lengths. Furthermore, regioselective control of product formation can be readily achieved through manipulation of base identity and reaction temperature conditions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
"Chemical ligation": a versatile method for nucleoside modification with boron clusters.Chemistry. 2008;14(34):10675-82. doi: 10.1002/chem.200801053. Chemistry. 2008. PMID: 18942698
-
Nucleoside-metallacarborane conjugates for base-specific metal labeling of DNA.Chemistry. 2007;13(1):311-8. doi: 10.1002/chem.200600740. Chemistry. 2007. PMID: 17103465
-
Aggregation behavior of nucleoside-boron cluster conjugates in aqueous solutions.Langmuir. 2008 Mar 18;24(6):2625-30. doi: 10.1021/la702852e. Epub 2008 Feb 8. Langmuir. 2008. PMID: 18257589
-
Chemical Synthesis of Acyclic Nucleoside Phosphonate Analogs Linked with Cyclic Systems between the Phosphonate and the Base Moieties.Curr Med Chem. 2020;27(35):5918-5948. doi: 10.2174/0929867326666190620100217. Curr Med Chem. 2020. PMID: 31250746 Review.
-
Boron-selective reactions as powerful tools for modular synthesis of diverse complex molecules.Chem Soc Rev. 2015 Dec 21;44(24):8848-58. doi: 10.1039/c5cs00338e. Epub 2015 Sep 22. Chem Soc Rev. 2015. PMID: 26393673 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

