Contribution of Enhanced Locoregional Control to Improved Overall Survival with Consolidative Durvalumab after Concurrent Chemoradiotherapy in Locally Advanced Non-Small Cell Lung Cancer: Insights from Real-World Data
- PMID: 38228082
- PMCID: PMC11261197
- DOI: 10.4143/crt.2023.1014
Contribution of Enhanced Locoregional Control to Improved Overall Survival with Consolidative Durvalumab after Concurrent Chemoradiotherapy in Locally Advanced Non-Small Cell Lung Cancer: Insights from Real-World Data
Abstract
Purpose: This study aimed to assess the real-world clinical outcomes of consolidative durvalumab in patients with unresectable locally advanced non-small cell lung cancer (LA-NSCLC) and to explore the role of radiotherapy in the era of immunotherapy.
Materials and methods: This retrospective study assessed 171 patients with unresectable LA-NSCLC who underwent concurrent chemoradiotherapy (CCRT) with or without consolidative durvalumab at Asan Medical Center between May 2018 and May 2021. Primary outcomes included freedom from locoregional failure (FFLRF), distant metastasis-free survival (DMFS), progression-free survival (PFS), and overall survival (OS).
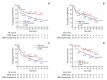
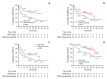
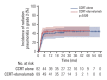
Results: Durvalumab following CCRT demonstrated a prolonged median PFS of 20.9 months (p=0.048) and a 3-year FFLRF rate of 57.3% (p=0.008), compared to 13.7 months and 38.8%, respectively, with CCRT alone. Furthermore, the incidence of in-field recurrence was significantly greater in the CCRT-alone group compared to the durvalumab group (26.8% vs. 12.4%, p=0.027). While median OS was not reached with durvalumab, it was 35.4 months in patients receiving CCRT alone (p=0.010). Patients positive for programmed cell death ligand 1 (PD-L1) expression showed notably better outcomes, including FFLRF, DMFS, PFS, and OS. Adherence to PACIFIC trial eligibility criteria identified 100 patients (58.5%) as ineligible. The use of durvalumab demonstrated better survival regardless of eligibility criteria.
Conclusion: The use of durvalumab consolidation following CCRT significantly enhanced locoregional control and OS in patients with unresectable LA-NSCLC, especially in those with PD-L1-positive tumors, thereby validating the role of durvalumab in standard care.
Keywords: Concurrent chemoradiotherapy; Durvalumab; Immunotherapy; Non-small-cell lung carcinoma; Radiotherapy.
Conflict of interest statement
Conflict of interest relevant to this article was not reported.
Figures
References
-
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–29. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

