Improving DNA nanostructure stability: A review of the biomedical applications and approaches
- PMID: 38228209
- PMCID: PMC11060068
- DOI: 10.1016/j.ijbiomac.2024.129495
Improving DNA nanostructure stability: A review of the biomedical applications and approaches
Abstract
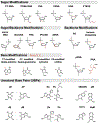
DNA's programmable, predictable, and precise self-assembly properties enable structural DNA nanotechnology. DNA nanostructures have a wide range of applications in drug delivery, bioimaging, biosensing, and theranostics. However, physiological conditions, including low cationic ions and the presence of nucleases in biological systems, can limit the efficacy of DNA nanostructures. Several strategies for stabilizing DNA nanostructures have been developed, including i) coating them with biomolecules or polymers, ii) chemical cross-linking of the DNA strands, and iii) modifications of the nucleotides and nucleic acids backbone. These methods significantly enhance the structural stability of DNA nanostructures and thus enable in vivo and in vitro applications. This study reviews the present perspective on the distinctive properties of the DNA molecule and explains various DNA nanostructures, their advantages, and their disadvantages. We provide a brief overview of the biomedical applications of DNA nanostructures and comprehensively discuss possible approaches to improve their biostability. Finally, the shortcomings and challenges of the current biostability approaches are examined.
Keywords: Biomedical applications; Biostability; DNA nanostructures; DNA nucleases.
Copyright © 2024 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



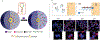

References
-
- Kuang H, Xu C, DNA-based chiral nanostructures, in: Chiral Nanomater. Prep. Prop. Appl, 2017: pp. 179–221. 10.1002/9783527682782.ch7. - DOI
-
- Sharma A, Vaghasiya K, Verma RK, Yadav AB, DNA nanostructures: Chemistry, self-assembly, and applications, Elsevier Inc., 2018. 10.1016/B978-0-323-51254-1.00003-8. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

