Amnesia after Repeated Head Impact Is Caused by Impaired Synaptic Plasticity in the Memory Engram
- PMID: 38228367
- PMCID: PMC10883615
- DOI: 10.1523/JNEUROSCI.1560-23.2024
Amnesia after Repeated Head Impact Is Caused by Impaired Synaptic Plasticity in the Memory Engram
Abstract
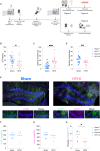
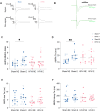
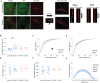
Subconcussive head impacts are associated with the development of acute and chronic cognitive deficits. We recently reported that high-frequency head impact (HFHI) causes chronic cognitive deficits in mice through synaptic changes. To better understand the mechanisms underlying HFHI-induced memory decline, we used TRAP2/Ai32 transgenic mice to enable visualization and manipulation of memory engrams. We labeled the fear memory engram in male and female mice exposed to an aversive experience and subjected them to sham or HFHI. Upon subsequent exposure to natural memory recall cues, sham, but not HFHI, mice successfully retrieved fearful memories. In sham mice the hippocampal engram neurons exhibited synaptic plasticity, evident in amplified AMPA:NMDA ratio, enhanced AMPA-weighted tau, and increased dendritic spine volume compared with nonengram neurons. In contrast, although HFHI mice retained a comparable number of hippocampal engram neurons, these neurons did not undergo synaptic plasticity. This lack of plasticity coincided with impaired activation of the engram network, leading to retrograde amnesia in HFHI mice. We validated that the memory deficits induced by HFHI stem from synaptic plasticity impairments by artificially activating the engram using optogenetics and found that stimulated memory recall was identical in both sham and HFHI mice. Our work shows that chronic cognitive impairment after HFHI is a result of deficiencies in synaptic plasticity instead of a loss in neuronal infrastructure, and we can reinstate a forgotten memory in the amnestic brain by stimulating the memory engram. Targeting synaptic plasticity may have therapeutic potential for treating memory impairments caused by repeated head impacts.
Keywords: amnesia; brain trauma; concussion; engram; head impact optogenetics.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
High-Frequency Head Impact Disrupts Hippocampal Neural Ensemble Dynamics.Front Cell Neurosci. 2022 Jan 18;15:763423. doi: 10.3389/fncel.2021.763423. eCollection 2021. Front Cell Neurosci. 2022. PMID: 35115908 Free PMC article.
-
Peripuberty Is a Sensitive Period for Prefrontal Parvalbumin Interneuron Activity to Impact Adult Cognitive Flexibility.Dev Neurosci. 2025;47(2):127-138. doi: 10.1159/000539584. Epub 2024 Jun 3. Dev Neurosci. 2025. PMID: 38830346 Free PMC article.
-
Long-Term Memory Engrams From Development to Adulthood.Hippocampus. 2025 Sep;35(5):e70032. doi: 10.1002/hipo.70032. Hippocampus. 2025. PMID: 40767385 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
Cited by
-
Memory engram synapse 3D molecular architecture visualized by cryoCLEM-guided cryoET.bioRxiv [Preprint]. 2025 Jan 12:2025.01.09.632151. doi: 10.1101/2025.01.09.632151. bioRxiv. 2025. PMID: 39829918 Free PMC article. Preprint.
-
Neurodegeneration in the cortical sulcus is a feature of chronic traumatic encephalopathy and associated with repetitive head impacts.Acta Neuropathol. 2024 Dec 6;148(1):79. doi: 10.1007/s00401-024-02833-8. Acta Neuropathol. 2024. PMID: 39643767 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
