The origin, evolution, and molecular diversity of the chemokine system
- PMID: 38228369
- PMCID: PMC10792014
- DOI: 10.26508/lsa.202302471
The origin, evolution, and molecular diversity of the chemokine system
Abstract
Chemokine signalling performs key functions in cell migration via chemoattraction, such as attracting leukocytes to the site of infection during host defence. The system consists of a ligand, the chemokine, usually secreted outside the cell, and a chemokine receptor on the surface of a target cell that recognises the ligand. Several noncanonical components interact with the system. These include a variety of molecules that usually share some degree of sequence similarity with canonical components and, in some cases, are known to bind to canonical components and/or to modulate cell migration. Whereas canonical components have been described in vertebrate lineages, the distribution of the noncanonical components is less clear. Uncertainty over the relationships between canonical and noncanonical components hampers our understanding of the evolution of the system. We used phylogenetic methods, including gene-tree to species-tree reconciliation, to untangle the relationships between canonical and noncanonical components, identify gene duplication events, and clarify the origin of the system. We found that unrelated ligand groups independently evolved chemokine-like functions. We found noncanonical ligands outside vertebrates, such as TAFA "chemokines" found in urochordates. In contrast, all receptor groups are vertebrate-specific and all-except ACKR1-originated from a common ancestor in early vertebrates. Both ligand and receptor copy numbers expanded through gene duplication events at the base of jawed vertebrates, with subsequent waves of innovation occurring in bony fish and mammals.
© 2024 Aleotti et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

















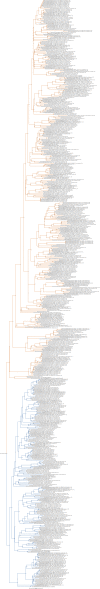












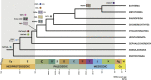
Similar articles
-
Evolution of endothelin receptors in vertebrates.Gen Comp Endocrinol. 2014 Dec 1;209:21-34. doi: 10.1016/j.ygcen.2014.06.028. Epub 2014 Jul 8. Gen Comp Endocrinol. 2014. PMID: 25010382 Review.
-
The endothelin system: evolution of vertebrate-specific ligand-receptor interactions by three rounds of genome duplication.Mol Biol Evol. 2009 Apr;26(4):783-99. doi: 10.1093/molbev/msp015. Epub 2009 Jan 27. Mol Biol Evol. 2009. PMID: 19174480
-
Evolution of developmental roles of Pax2/5/8 paralogs after independent duplication in urochordate and vertebrate lineages.BMC Biol. 2008 Aug 22;6:35. doi: 10.1186/1741-7007-6-35. BMC Biol. 2008. PMID: 18721460 Free PMC article.
-
Origin of Nogo-A by domain shuffling in an early jawed vertebrate.Mol Biol Evol. 2011 Apr;28(4):1363-70. doi: 10.1093/molbev/msq313. Epub 2010 Nov 22. Mol Biol Evol. 2011. PMID: 21098000
-
Evolution and function of chemokine receptors in the immune system of lower vertebrates.Eur J Immunol. 2013 Jul;43(7):1686-92. doi: 10.1002/eji.201343557. Eur J Immunol. 2013. PMID: 23719857 Review.
Cited by
-
CXCL17 is a proinflammatory chemokine and promotes neutrophil trafficking.J Leukoc Biol. 2024 May 29;115(6):1177-1182. doi: 10.1093/jleuko/qiae028. J Leukoc Biol. 2024. PMID: 38298146 Free PMC article.
-
A lymphocyte chemoaffinity axis for lung, non-intestinal mucosae and CNS.Nature. 2024 Nov;635(8039):736-745. doi: 10.1038/s41586-024-08043-2. Epub 2024 Sep 18. Nature. 2024. PMID: 39293486 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
