Primary and secondary defects of the thymus
- PMID: 38228406
- PMCID: PMC10950553
- DOI: 10.1111/imr.13306
Primary and secondary defects of the thymus
Abstract
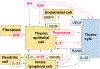
The thymus is the primary site of T-cell development, enabling generation, and selection of a diverse repertoire of T cells that recognize non-self, whilst remaining tolerant to self- antigens. Severe congenital disorders of thymic development (athymia) can be fatal if left untreated due to infections, and thymic tissue implantation is the only cure. While newborn screening for severe combined immune deficiency has allowed improved detection at birth of congenital athymia, thymic disorders acquired later in life are still underrecognized and assessing the quality of thymic function in such conditions remains a challenge. The thymus is sensitive to injury elicited from a variety of endogenous and exogenous factors, and its self-renewal capacity decreases with age. Secondary and age-related forms of thymic dysfunction may lead to an increased risk of infections, malignancy, and autoimmunity. Promising results have been obtained in preclinical models and clinical trials upon administration of soluble factors promoting thymic regeneration, but to date no therapy is approved for clinical use. In this review we provide a background on thymus development, function, and age-related involution. We discuss disease mechanisms, diagnostic, and therapeutic approaches for primary and secondary thymic defects.
Keywords: AIRE; DiGeorge syndrome; FOXN1; T-cell reconstitution; athymia; immune restoration; immunodeficiency; immunosenescence; thymic atrophy; thymic epithelial cells; thymic involution; thymic regeneration; thymopoiesis; thymus; thymus transplantation.
Published 2024. This article is a U.S. Government work and is in the public domain in the USA.
Conflict of interest statement
Figures


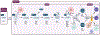
References
-
- DiGeorge A. Discussions on a new concept of the cellular base of immunology. J Pediatr. 1965;67:907.
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

