Inflammatory recruitment of healthy hematopoietic stem and progenitor cells in the acute myeloid leukemia niche
- PMID: 38228679
- PMCID: PMC10997516
- DOI: 10.1038/s41375-024-02136-7
Inflammatory recruitment of healthy hematopoietic stem and progenitor cells in the acute myeloid leukemia niche
Abstract
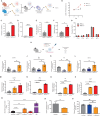
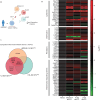
Inflammation in the bone marrow (BM) microenvironment is a constitutive component of leukemogenesis in acute myeloid leukemia (AML). Current evidence suggests that both leukemic blasts and stroma secrete proinflammatory factors that actively suppress the function of healthy hematopoietic stem and progenitor cells (HSPCs). HSPCs are also cellular components of the innate immune system, and we reasoned that they may actively propagate the inflammation in the leukemic niche. In two separate congenic models of AML we confirm by evaluation of the BM plasma secretome and HSPC-selective single-cell RNA sequencing (scRNA-Seq) that multipotent progenitors and long-lived stem cells adopt inflammatory gene expression programs, even at low leukemic infiltration of the BM. In particular, we observe interferon gamma (IFN-γ) pathway activation, along with secretion of its chemokine target, CXCL10. We show that AML-derived nanometer-sized extracellular vesicles (EVAML) are sufficient to trigger this inflammatory HSPC response, both in vitro and in vivo. Altogether, our studies indicate that HSPCs are an unrecognized component of the inflammatory adaptation of the BM by leukemic cells. The pro-inflammatory conversion and long-lived presence of HSPCs in the BM along with their regenerative re-expansion during remission may impact clonal selection and disease evolution.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: A Cancer J Clin. 2022;72:7–33. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

